General organic chemistry Questions and Answers

Organic Chemistry
General organic chemistryHow many chiral carbon atoms does the compound below contain

Organic Chemistry
General organic chemistryList all possible values of the magnetic quantum number m for a 4x electron M 0 an S

Organic Chemistry
General organic chemistryWhat reaction mechanism would be used for this reaction It results in an epoxide but what reaction method would be used 2 Describe the reaction mechanism of the following reaction 2 Describe the reaction mechanism of the following reaction OH TsCl OH pyridine OH OTS NaOH C

Organic Chemistry
General organic chemistryExamine the radicals below closely Draw out and consider any resonance structures each might have I W III Which radical is the most stable Select Select Which radical is the least stable 1 DY II IV

Organic Chemistry
General organic chemistryA A main group element with the valence electron configuration 3s 3p is in periodic group It forms a monatomic ion with a charge of B A main group element with the valence electron configuration 2s22p is in periodic group It forms a monatomic ion with a charge of

Organic Chemistry
General organic chemistryName the alkene below Hint make sure to include all needed stereo descriptors without using parentheses

Organic Chemistry
General organic chemistryQuestion 35 1 point Methotrexate image below is classified as an antimetabolite Methotrexate most closely resembles which endogenous compound NH M N avoi HO O5 hydroxytryptamine Aspartic acid Histamine Folate Epinephrine Next Page Page 3
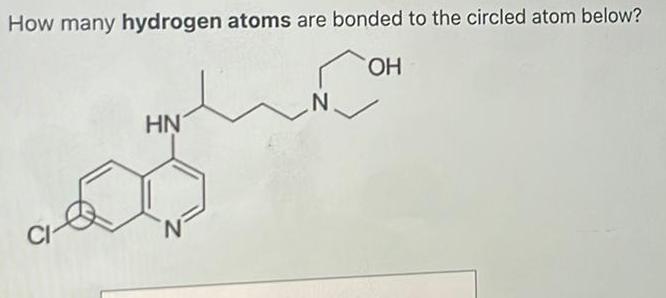
Organic Chemistry
General organic chemistryHow many hydrogen atoms are bonded to the circled atom below OH CI HN N N

Organic Chemistry
General organic chemistrygiven the major products for each of the following reactions or sequences of reaction show all relevant stereochemistry 4 Br 1 Mg ether 2 CO 3 H3O

Organic Chemistry
General organic chemistryThe enthalpy change for the following reaction is 121 kJ Using bond energies estimate the C H bond energy in CH4 g CH4 g Cl g CH3CI g HCl g kJ mol

Organic Chemistry
General organic chemistryMatch the reagents with each step Drag and drop options on the right hand side to reorder and match with items on the left Reordering may cause items on the right hand side to swap positions Step 1 Step 2 Step 3 Step 4 Step 5 CH3CH Li THF H O NaNHz NH3 epoxyethane CH 2 CH3CH Br III III

Organic Chemistry
General organic chemistryBr Draw the curved arrow mechanism for the reaction between the aldehyde and propyl bromide including the final product Be sure to include nonzero formal charges and lone pair electrons on all appropriate atoms Draw the resonance structure of the enolate ion only Do NOT include curved arrows in this box need help with 3rd box only thx

Organic Chemistry
General organic chemistryWhat is the molar mass of a gas if 1 15 g of the gas nas STP Your answer should have three significant figures Round to the nearest whole number

Organic Chemistry
General organic chemistryWhich one of the following is the general form of the Lewis dot formula for chlorine X

Organic Chemistry
General organic chemistryLabel all acids and bases and conjugate acids and bases in the reaction below O base IZ conjugate base acid conjugate acid ZO

Organic Chemistry
General organic chemistryIf the temperature of a reaction is increased how will this affect the reaction rate according to the equation shown below if the concentration of reactants is not changed Ea k Ae RT Select one O reaction rate will increase reaction rate will decrease reaction rate will stay the same

Organic Chemistry
General organic chemistryMatch the reagent to the steps for the synthesis of decane from acetylene Drag and drop options on the right hand side to reorder and match with items on the left Reordering may cause items on the right hand side to swap positions Step 1 Step 2 Step 3 Step 4 Step 5 H Pt CH3CH CH CH Br NaNH3 NaNH2 NH3 CH3CH CH CH Br NaNH2 NH3 H Lindlar s catalyst III

Organic Chemistry
General organic chemistryFor each pair of covalently bonded atoms choose the one expected to have the higher bond energy The strength of a covalent bond depends upon the size of the atoms and the bond order In general short bonds are strong bonds A C O B C 0 C C N D C N A B les i C D ded for this question

Organic Chemistry
General organic chemistryDraw the conjugate acid and conjugate base for the reaction below Which acid is the strongest acid in the equilibrium reaction below Also on which side is the equilibrium residing i e the side on which components that have the higher concentration once equilibrium is reached Select all answers that apply Select one or more conjugate acid conjugate base The equilibrium is residing on the product side strongest acid is the conjugate base The equilibrium is residing on the reactant side strongest acid is the conjugate acid strongest acid is the acid strongest acid is the base

Organic Chemistry
General organic chemistryFill in the orbital energy diagram for sulfur E 3s 2s 1s 3p 2p

Organic Chemistry
General organic chemistryThe length of a covalent bond depends upon the size of the atoms and the bond order For each pair of covalently bonded atoms choose the one expected to have the shorter bond length N N or N N Use the References to access important values if needed for th CEN or C N

Organic Chemistry
General organic chemistryIf the compound below were reacted with H and Pd C which major product would be obtained Select one O 0 0 Br Br HO Br Br Br Br Br Br

Organic Chemistry
General organic chemistryR Alkyne MOFO A B C N D Azide III N Azide Using the image above follow the arrow pushing mechanism of the general click reaction shown to predict the product formed when Professor Carolyn Bertozzi s strained alkyne MOFO reacts with an azide Click Reaction Compound a Compound b Click Reaction Compound c Triazole No reaction occurs

Organic Chemistry
General organic chemistryWhat is the major product formed when hept 1 yne is treated with two equivalents of HBr A B C 1 1 dibromoheptane 2 2 dibromoheptane 1 2 dibromoheptane

Organic Chemistry
General organic chemistry1 2 A B 3 4 5 6 7 H J 8 9 F 10 11 12 13 H 14 15 16 17 CD G 18 E

Organic Chemistry
General organic chemistryWhat product is formed in the following reaction A Compound a B C Compound b Compound c Br Br a H3C C C CH3 excess Br CCI4 CI Br b H3C C C CH3 CI Br c H3C C C CH

Organic Chemistry
General organic chemistryWhat is the product formed in the following A Compound 1 B 1 H3C Compound 2 H Br CH3 2 eq HBr 2 H3C Br Br CH3

Organic Chemistry
General organic chemistryWhat is the keto tautomer of the following enol A B C Compound a Compound b Compound c D Compound d OH 11

Organic Chemistry
General organic chemistryWhich chair conformation is the least stable depiction of the compound drawn below OH Select one OH ZOH 7 OH MOH

Organic Chemistry
General organic chemistry2 A 3 4 5 6 7 8 9 10 11 12 16 F 13 14 15 16 H G 17 CD 18 Choose the correct letter that corresponds Choices Lanthanoid Series Metalloid Carbon Alkaline Earth Halogens Transition Metal Noble Gas Boron Group Actinoid Series Oxygen Group Alkali Metals In addition Label Hydrogen Shade a period Shade family group 4B

Organic Chemistry
General organic chemistry1 Atomic P 2 A Hydrogen E N I Atomic P 3 4 5 6 7 8 9 10 11 12 H FL Beryllium E 13 14 15 16 17 CD N G 18 E Atomic P E Fluorine N Atomic P E Lanthanoid Series Metalloid Carbon Alkaline Earth Choose the correct letter that corresponds Choices Halogens Transition Metal Noble Gas Boron Group Actinoid Series Argon Oxygen Group Alkali Metals N In addition Label Hydrogen Shade a period Shade family group 4B

Organic Chemistry
General organic chemistryHow many electron push arrows at a minimum are needed to convert the resonance structure on the left to the resonance structure on the right O O

Organic Chemistry
General organic chemistryand number the parent as learned in class Draw 4 ethyl 2 2 3 5 tetramethylnonane How many H atoms are bonded to carbon 2

Organic Chemistry
General organic chemistrySmol otod 3 For the reaction whose balanced equation is as follows find the number of grams of 12 that will be formed when 300 0 g of bromine 2KI Br2 2KBr I react 38 habivore DOW amaldo9 z20M 220M enditesup glavello si to rope wank MAJAS O BA JAWOST emo HA baar to emong 0 ad tr 4 For the reaction whose balanced equation is as follows find the number of grams of sodium that must react to produce 42 0 grams of sodium oxide 4Na O Na O Nazo taum noitonen antti tapan taunt naponby to mong yoom world HM binommo to anione o soubor 5 For the reaction whose balanced equation is as follows find how many grams of zinc phosphate will be produced by the reaction of 5 00 grams of ammonium phosphate 3 ZnCl2 2 NH4 3PO4 Zn3 PO4 2 6NH4Cl muninu to amo Cato 220mA SwoMS to

Organic Chemistry
General organic chemistryWhat type of strain is depicted in the structure below Select one O Baeyer or angle strain O Prelog or transannular strain Pitzer or torsions

Organic Chemistry
General organic chemistryPlease select all functional groups FGs that are found in the compound below Note incorrect answers Time N Select one or more O alkyl halide alkene alkyne alcohol ether thiol sulfide thioether arene amine aldehyde ketone carboxylic acid acyl halide carboxylic acid anhydride ester OH thioester

Organic Chemistry
General organic chemistryA potential drug needs to be able to bind to a Zn metalloenzyme in order to achieve the desired potency Which functional group might be the most useful for zinc binding O hydroxyl benzene ring ether O thiol

Organic Chemistry
General organic chemistryDraw the major product of this SN1 reaction Ignore any inorganic byproducts Br NaCl acetone H O


Organic Chemistry
General organic chemistryClick in the middle of each cell in the table below that contains a basic pH value pH 2 27 pH 4 21 pH 8 89 pH 12 6 pH 14 8 pH 0 56 pH 3 99 pH 6 5 pH 07 45 pH 978 pH 5 61 pH 911 7

Organic Chemistry
General organic chemistry1 Which molecule has the R configuration A OH B C HC HC A C H CC HO C CH OH H H CH3 COH H CH3 CH3 CH3 CH3 CH3 18 D CH3 CH3 H CH 6 Which Newman projection represents the most stable configuratic CH3 2CHCH CH3 2 HC HO CH OH x HC 031 B D CO H OH CH3 1010 9 H CH3 H CE CH3 CH3 CH3 H H CH3 CH3 H CU CH3 CH3

Organic Chemistry
General organic chemistryIn the following molecule identify functional groups that the two molecules have IN COMMON 000 alcohol ether amine aldehyde ketone carboxylic acid ester amide aromatic ring HOI HN CH3 CH 3 NH CH N Methylphenethylamine

Organic Chemistry
General organic chemistryPredict the products If no H 1 atm Pd C 1 0 CH3 products then NO REACTION

Organic Chemistry
General organic chemistryWhich compound is NOT a possible product of the reaction shown hv Cl FILCI CI I B CI EC CI IV CI CI V F

Organic Chemistry
General organic chemistryHow many polar bonds does the molecule below contain hint not all bonds may be drawn N H

Organic Chemistry
General organic chemistryta Possible reagents A BH3 THF H O NaOH B Na Cr O7 H SO4 C SOCI D LIAIH4 E PCC F Mg ether CH3CHO G H H O Step 1 Select 1 ti 2 3 Step 2 Select Step 3 Select

Organic Chemistry
General organic chemistryWhen the compound below is treated with NaOH which of the following compounds would be the expected major product Select one O Br OH OH

Organic Chemistry
General organic chemistryIn the reaction of Cl2 with ethane and UV light which of the following reactions would be a chain termination event s I Cl CH3 CH3 CH3 CH2 Cl H II Cl CH3 CH3 CH3 H2C HCI III Cl CH3 H2C CH3 CH2 Cl IV Cl2 CH3 H2C CH3 CH2 Cl Cl V Cl2 UV light C 1 Cl OA reaction V B reactions I and IV C reactions III and IV D reactions I and II E reaction III

Organic Chemistry
General organic chemistryWhat is the relationship of the two molecules drawn below Select one O diastereomers O conformers Oenantiomers O constitutional isomers same

Organic Chemistry
General organic chemistryWhat is the configuration of the circled carbon atom below vel Select one OR configuration O carbon atom is not chiral and has therefore no configuration OS configuration