General organic chemistry Questions and Answers

Organic Chemistry
General organic chemistryThe name 4-ethylpentane is not an accepted name for the compound described. What
would be the correct name for this compound?
1. 2-ethylpentane
2. 4-methylhexane
3. 1-ethyl-1-methylbutane
4. 3-methylhexane
5. n-heptane

Organic Chemistry
General organic chemistryThe pk, of acetic acid, CH3COOH, is 4.76. What is the value of the equilibrium constant Keq, for the following equilibrium? Show work using the equation function from the menu above
CH₂COOH + H₂O
H₂00+ CH₂COO

Organic Chemistry
General organic chemistryUsing the periodic table, what do you know about Indium?
It is a large metal and likely to react with nonmetals.
It is a small metal and unlikely to react with anything else
It is a small nonmetal and likely to react with other metals
It is a large nonmetal and unlikely to react with other metals
It is a large nonmetal and likely to react with other nonmetals

Organic Chemistry
General organic chemistryUsing the periodic table, what do you know about Rubidium?
It has a high electronegativity and a high reactivity
It is a small atom and has a low electronegativity
It has a low electronegativity and has a high reactivity
It has a low electronegativity and has a low reactivity
It has a high electronegativity and a low reactivity
Organic Chemistry
General organic chemistryWhich of these is most likely to react with
Strontium?
Selenium
Xenon
Potassium
None of these
Argon
Krypton

Organic Chemistry
General organic chemistryHow will an LOL diagram show energy being conserved?
Energy will be destroyed.
There will be more energy in the reactants than products
All energy will be accounted for
There will be more energy in the products than in the reactants
The energy won't change

Organic Chemistry
General organic chemistryWhat is true about nuclear fission?
Two small nuclei come together.
It is not used in atomic bombs.
It is occuring in the sun.
Scientists have not yet been able to harness nuclear fission to create electricity.
It occurs when a large unstable nucleus splits into smaller nuclei.

Organic Chemistry
General organic chemistryThe effective nuclear charge, Zeff, for a valence electron can be approximated using the core charge of the atom; that is, the net charge of the nucleus and the inner (nonvalence) electrons. Determine the core charge for an atom of Ar.

Organic Chemistry
General organic chemistryIf 24g of hydrogen gas is fully reacted with excess carbon, how many particles, in moles of benzene
(C6H6) will form?
6C(s) + 3H2(g)--C6H6
2.3mol Benzene
4mol Benzene
24mol Benzene
312g Benzene
12mol Benzene

Organic Chemistry
General organic chemistrySulfur has how many valence electrons? What is it's charge when it is stable?
2,6
2,2
6, -6
6,6
6,2
6, -2

Organic Chemistry
General organic chemistryUse the pka table to select a base to deprotonate nitrous acid (HNO₂) pka = 3.35 Write the acid base reaction Calculate the equilibrium constant to demonstrate that you selected a good base. Write the curved arrow mechanism in both directions

Organic Chemistry
General organic chemistryWrite the curved arrow mechanism for the dissociation of sulfuric acid in water (show all bonds and lone pairs, i.e. Lewis structures) Which will be more abundant at 25 °C, sulfuric acid or hydronium ion? Why? What type of acid base reaction is this? Define the role of water, i.e Lewis base, Brønsted-Lowry acid, etc.

Organic Chemistry
General organic chemistryIf 109g of water was created in the following equation, how much energy was released?
CH4(g)+202(g) CO2(g) +2H₂O) AH = -890.8
Round to two decimal places.

Organic Chemistry
General organic chemistryWhich of these is the least electronegative?
Sodium
Rubidium
Potassium
Hydrogen
Lithium

Organic Chemistry
General organic chemistryIf a person has 621mL of a 4.2M KCI solution. How many moles of KCI would be in the solution? (1000mL-1L) Round to two decimal places.

Organic Chemistry
General organic chemistryWhat is true about an endothermic reaction?
They often feel hot to the touch.
More bonds are being broken than formed
More bonds are being formed than broken
Equal amounts of bonds are being broken and formed
No bonds are being broken or formed

Organic Chemistry
General organic chemistryIf 3mol of solid carbon is reacted with 3mol of hydrogen gas, what mass of benzene (C6H6) is likely to form?
6C(s) + 3H2(g) --> C6H6(1)
234g Benzene
0.5g Benzene
78g Benzene
39g Benzene
24.2g Benzene

Organic Chemistry
General organic chemistryIf a person has 162mol of K₂O, what mass, in grams, of K₂O doo they have? Round to two decimal places.

Organic Chemistry
General organic chemistryThe molarity (M) of the Ca(NO3)2 solution when 61.3 mL react with 46.2 mL of 5.2 M Na3PO4

Organic Chemistry
General organic chemistryHow would you prepare the following two compounds starting from benzene? (Proper sequence of steps with proper reagents necessary for each step must be given)
HO.
-NH₂
HO-
-NH₂

Organic Chemistry
General organic chemistryYou have 125.0 mL of a solution of H3PO4, but you don't know its concentration. If you titrate the solution with a 4.56 M solution of NaOH and reach the endpoint when 134.1 mL of the base are added, what is the concentration of the acid?

Organic Chemistry
General organic chemistryWhich of the following solutions could be used to titrate a HCl solution with an unknown concentration if the titration was using a pH indicator?
1.5M HBr solution
2M NaCl solution
5.2M LICI solution
2M NaOH solution
1.68M water solution

Organic Chemistry
General organic chemistryWhy does a balanced equation represent mass
being conserved?
Balancing an equation results in different amounts of mass in the reactants and products
Balancing a reaction creates mass.
Balancing a reaction results in the same molecules being present in the reactants and products.
Balancing a reaction results in the same number and type of atom being present in the reactants and products.
Balancing a reaction changes the type of substance being reacted, so mass is neither created nor destroyed.

Organic Chemistry
General organic chemistryWhat is the chemical formula for Aluminum Bisulfate?
AIBI3
Al2(SO4)3
AI(HSO4)3
AI(HSO4)2
OAIHSO4

Organic Chemistry
General organic chemistryAccording to IUPAC, when counting the carbons in a branched hydrocarbon chain you would use the shortest possible chain.
True
False

Organic Chemistry
General organic chemistryWhich of the following statements does not characterize an oxidizing agent?
The oxidation number of an oxidizing agent decreases
An oxidizing agent gains electrons
An oxidizing agent causes another species to be oxidized
An example of a good oxidizing agent is an alkali metal, such as sodium (Na)

Organic Chemistry
General organic chemistryM/ Ch. Many alcohols are made by the addition reaction of what two types of compounds?
water and alkenes
alkanes and alkenes
Oesters and ethers
water and alkanes

Organic Chemistry
General organic chemistryM/Ch. In a hydration reaction, an alkene is converted to what type of compound?
an alkyne
an alcohol
an amide
an aldehyde

Organic Chemistry
General organic chemistryWhich species is oxidized in the following reaction?
2Hg²+ + N₂H4 → 2Hg + N₂ + 4H
This is a reduction reaction not an oxidation reaction
Nitrogen
Hydrogen
Mercury

Organic Chemistry
General organic chemistryWhat are the intermolecular forces experienced by the interaction of the following molecules? NH3 and C6H6 (benzene)
only dispersion forces
dipole-induced dipole forces and dispersion forces
ion-induced dipole forces and dispersion foces
dipole-dipole and dispersion forces
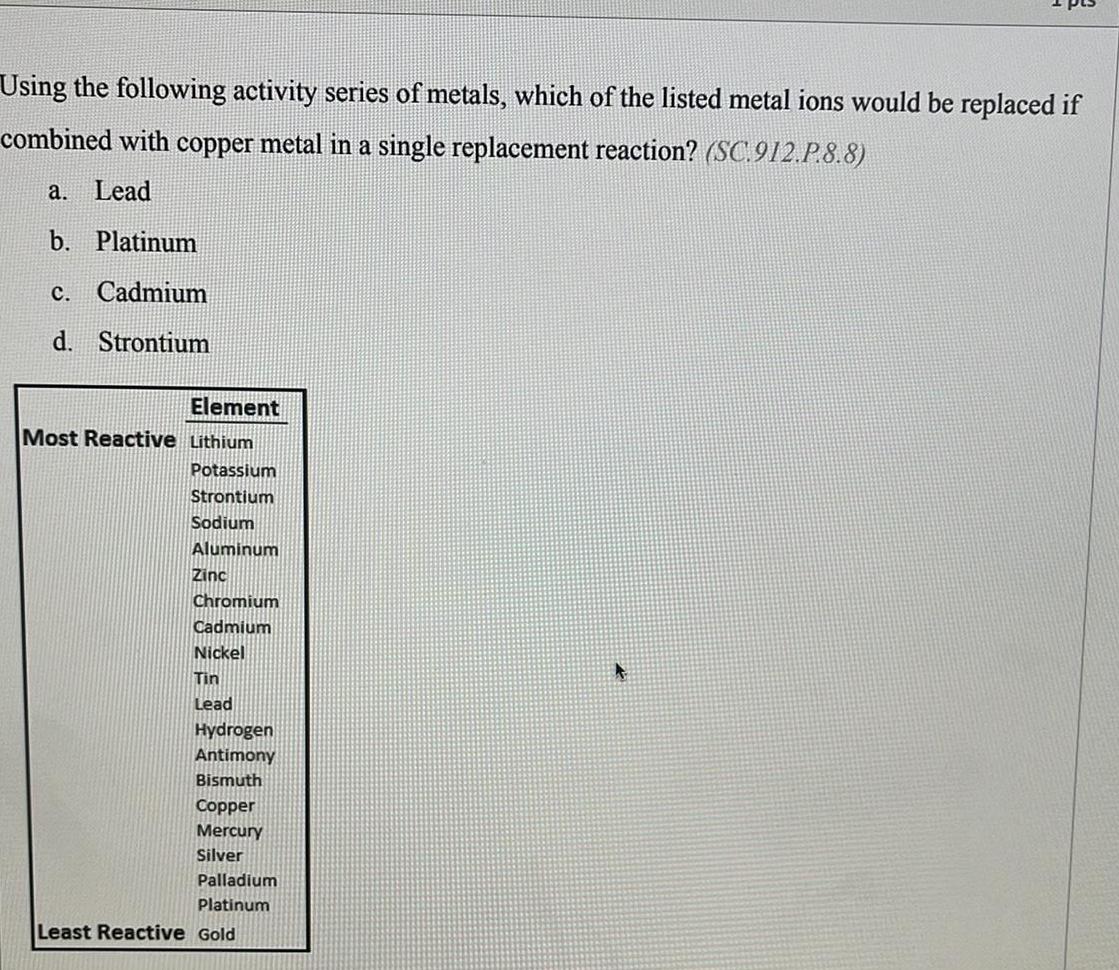
Organic Chemistry
General organic chemistryUsing the following activity series of metals, which of the listed metal ions would be replaced if combined with copper metal in a single replacement reaction?
a. Lead
b. Platinum
c. Cadmium
d. Strontium

Organic Chemistry
General organic chemistryIdentify the best reagents to achieve the following transformation:
1. conc. H₂SO4, heat; 2. HBr, ROOR
1. NaOEt; 2. HBr, ROOR; 3. t-BuOK
1. NaOEt; 2. BH3 THF; 3. NaOH, H₂O2
1. conc. H₂SO4, heat; 2. HBr

Organic Chemistry
General organic chemistryMetal Specific Heat (J/g°C)
Calcium 0.647
Iron 0.449
Silver 0.235
Gold 0.129
Ten grams of all four substances above are pulled from the same container of hot oil with a temperature of 250°C. Determine which substance will cool off fastest and explain how you know.

Organic Chemistry
General organic chemistryWrite balanced equations for the ionization of each of the
following carboxylic acids in water.
acetic acid

Organic Chemistry
General organic chemistryWhich of the following statements is (are) true for the compound (R)-2-butanol?
A) This compound is chiral.
B) This compound is optically active.
C) This compound has an enantiomer
D) all of the above
E) none of the above

Organic Chemistry
General organic chemistryGive the products for each of the following processes
Drag the appropriate items to their respective bins.
Product of
hydrogenation
palmitic acid
Br₂
0₂ alkane
Product of
hydrolysis
glycerol
the potassium salts of the fatty acids
Product of
saponification
ethylene glycol
Not a product of
these reactions
Reset

Organic Chemistry
General organic chemistryA compound is found to contain 45.71 % oxygen and 54.29 % fluorine by mass. What is the empirical formula for this compound?

Organic Chemistry
General organic chemistryThe saponification of an ester produced
isopropanol and sodum octanoate. What
ester underwent hydrolysis to give these
products
View Available Hint(s)
isopropyl octanoate
Isopropyl octanol
isopropyl octanoic acid)
octyl isopropanoate

Organic Chemistry
General organic chemistryA compound is found to contain 6.360 % silicon, 36.18 % bromine, and 57.46 % iodine by mass. What is the empirical formula for this compound? To answer the question, enter the elements in the order presented above.

Organic Chemistry
General organic chemistryEnter your answer in the provided box.
How many degrees of unsaturation are present in C8H₂CIO?
degrees of unsaturation

Organic Chemistry
General organic chemistryWhat is the total number of oxygen atoms present in Mg(CIO3)2?
2
6
3
5

Organic Chemistry
General organic chemistryShow the calculation of osmolarity for each of the following solutions. 3 points
a. 0.75 M magnesium nitrate solution has osmolarity of
b. 1.5 M sucrose solution has an osmolarity of
c. 0.952 moles of a copper (II) chloride in 806 mL of water has an osmolarity of

Organic Chemistry
General organic chemistryIncreasing pressure speeds up the rate of a chemical reaction because particles are compacted in a smaller volume, therefore increasing particle collisions.
Pressure only affects which phase?
aqueous
gas
liquid
solid

Organic Chemistry
General organic chemistryEnter the balanced chemical equation for the reaction of each of the following carboxylic acids with KOH
3-methylpentanoic acid (CH₂CH₂CH(CH3)CH₂COOH)
Express your answer as a chemical equation. Assume that there is no dissociation (.e., enter only whole compounds, not i

Organic Chemistry
General organic chemistryApply the Pauli exclusion principle.
(a) Indicate which orbitals in the following list fill before the 4p orbitals.
3d _4s _4d_ 5s
(b) What is the maximum number of electrons in the 4f subshell?
(c) An electron in an orbital has the following quantum numbers: n = 3, l = 2, m₁ = 0,
and ms = +1/2.
Identify the type of orbital.
What is the direction of the electron spin?

Organic Chemistry
General organic chemistryEstimate A proton's mass is estimated to be 1.6726 × 10-24 g, and the mass of an electron is estimated to be 9.1093 × 10-28 g. How many times larger is the mass of a proton compared to the mass of an electron?

Organic Chemistry
General organic chemistryCalculate ΔH for the reaction: C₂H4 (g) + H₂ (g) → C₂H6 (g), from the following data.
C₂H4 (g) +3O₂ (g) → 2 CO₂ (g) + 2 H₂O (l)
C2H6 (g) + 3 1/2O, (g) → 2 CO2 (g)+ 3 H2O (l)
H₂(g) + 1/2O₂(g) → H₂O (l)

Organic Chemistry
General organic chemistryIf CO₂ is being produced in the solution at a faster
rate than H₂CO3, then the rate of the
reaction is faster than the rate of the
reaction.
a. forward / reverse
b. reverse / forward
c. neither A nor B is correct

Organic Chemistry
General organic chemistryA mixture of equal amounts of two enantiomers
A) is called a racemic mixture
B) is optically inactive
A) is called a C) implies that the enantiomers are meso forms
D) both A and B
E) none of the above

Organic Chemistry
General organic chemistryPhotosynthesis uses 670-nm light to convert
CO₂ and
H₂O into glucose and
O₂. Calculate the frequency of this light.
Frequency: Hz