General organic chemistry Questions and Answers

Organic Chemistry
General organic chemistryAt the gym, a man pulls a bar on a machine that works the muscles of the upper back. It takes him 0.5 seconds to raise 30 kilograms of weights a vertical distance of 0.5 meters.
Which of these exerts the same power output? (Estimate g as 10 m/s2.)
A Alifting 25 kilograms a distance of 2.4 meters in 2.0 seconds
B lifting 45 kilograms a distance of 2.4 meters in 3.0 seconds
C leg pressing 45 kilograms a distance of 0.5 meters in 0.5 seconds
D bench pressing 30 kilograms a distance of 0.5 meter in 1.5 seconds

Organic Chemistry
General organic chemistryAt equilibrium, the total amount of the product(s)
is always greater than the total amount of the reactan
is always less than the total amount of the reactants
is always equal to the total amount of the reactants
may be equal to, greater than, or less than the total am

Organic Chemistry
General organic chemistryIron (III) metal is placed in a container filled with chlorine gas. When the equation is balanced, what is the coefficient for chlorine?
a) 3
b) 1
c) 4
d) 2

Organic Chemistry
General organic chemistryConsider the following equation: 6Li + N₂ → 2Li3N
a. If 21.48 mol of lithium react with 12.8 mol of nitrogen, which is the limiting reactant? (1)
b. How many moles of lithium nitride can be produced? (1)
c. How many moles of the excess reactant are left over? (1)

Organic Chemistry
General organic chemistryFind the partial pressure of carbon dioxide in a gas mixture with a total pressure of 30.5 kPa if the partial pressures of the other two gases in the mixture are 16.5 kPa and 3.7 kPa.
Show work below:

Organic Chemistry
General organic chemistryAll of the processes you'll see in this assessment takes place in liquid chloroform, CHCl3. Draw a full Lewis structure for chloroform, showing all atoms, bonds, and lone pairs. Also annotate your structure with δ+ and δ- signs for partial charges.

Organic Chemistry
General organic chemistryIn each of the following reactions, assign oxidation states to each of the atoms to identify the substance that is oxidized (loses electrons) and the substance that is reduced (gains electrons).
a) Cr₂O3 + 2 Al → Al₂O3 + 2 Cr
b) 3 H₂+ Fe₂O3 → 2 Fe + 3 H₂O
c) 6 Ag + 3 HS- + 2 CrO4+ 5H₂O → 3 Ag₂S + 2 Cr(OH)3 + 7 OH-

Organic Chemistry
General organic chemistryFor an exothermic reaction that is thermodynamically favorable which of the following must be true?
A) ΔG must be positive
B) ΔS must be positive
C) ΔS must be negative
D) None of the above must be true

Organic Chemistry
General organic chemistryFor a the following reaction ΔH = 6.2 kJ and ΔS = -33 J/K. Under what temperature and condition is the reaction spontaneous?
A) WhenT< -210K
B) WhenT< 210K
C) The reaction is not spontaneous at any temperature.
D) WhenT> -210K

Organic Chemistry
General organic chemistryAspirin (C9H8O4) if formed from the reaction of salicylic acid (C7H603) with liquid acetic acid according to the equation below. After the reaction the water is evaporated and solid aspirin is collected. How many grams of aspirin could be prepared by mixing 25.0 ml of 0.10 M C₂H603 with 5.00 ml of acetic acid (d=1.05 g/ml)
C7H6O3(aq) + HC2H3O2(l)→ C9H8O4(aq) + H₂O(l)

Organic Chemistry
General organic chemistryNew concept cars in the Detroit Auto Show feature engines that burn hydrogen gas in air to produce water vapor. Suppose that fuel tank contains 150. L of H: gas at 20.0 atm pressure at 25 °C. If all of this gas is burned, what mass of water vapor is produced?
Write a balanced chemical equation for the reaction.
Given: Unknown:
Show work below:

Organic Chemistry
General organic chemistryThe following successive ionization energies correspond to an element in the third row of the periodic table:
I1 = 786.3 kJ/mol,
I2=1,580 kJ/mol,
I3= 3,230 kJ/mol,
I4=4,360 kJ/mol,
I5 = 16,000 kJ/mol, and
I6=20,000 kJ/mol.
Based on this pattern of ionization energies, identify the element.
A. Mg
B. Al
C. Si
D. P

Organic Chemistry
General organic chemistryDetermine the volume of oxygen gas (in L) needed to completely combust 0.202 L of butane gas (C4H10) at constant temperature and pressure according to the following equation:
2C4H10(g) + 13O2(g) -> 8CO2(g) + 10H₂O(l)

Organic Chemistry
General organic chemistryAnswer the following questions for the reaction below at 298K
C₂H₂(g) + 4F2(g) <-> 2CF4(g) + H₂(g) ΔH = -93.3 kJ
a) Predict the sign of the entropy change, ΔS for the reaction. Explain the basis for your prediction.
b) If the temperature at which the reaction occurs is increased to 500K, the entropy, ΔS at is -358 J/K.
i) Calculate ΔG and explain if the reaction is spontaneous at this temperature.
ii) Calculate the equilibrium constant, Keq at this temperature.
iii) Is the K value you calculated consistent with the value of ΔG for the reaction? Explain.

Organic Chemistry
General organic chemistryRank the following molecules in order from least soluble in water to most soluble in water.
Cyclohexanol, 2-decanol, methanol

Organic Chemistry
General organic chemistryFor the reaction energy diagram shown below, which of the following statements is true?
A catalyst could make the forward reaction more exergonic.
According to the Hammond postulate, B resembles C in structure more than B resembles A in structure.
The equilibrium constant for the forward reaction is greater than 1.
The reaction mechanism has four elementary steps.
The reverse reaction is endergonic.

Organic Chemistry
General organic chemistryDraw all staggered and eclipsed Newman projections of the compound below rotating a full 360°. Calculate the energy of each conformation using the given values. To save space when drawing, you may abbreviate the t- butyl group as TB. Circle the conformation(s) with the highest energy. You may start with the conformation drawn below (15 pts).

Organic Chemistry
General organic chemistrySynthesis of a compound in an achiral environment which has enantiomers results in a racemic mixture. Which of the following accounts for this observation?
Enantiomeric transition state
Magnitude of the rate constants
Magnitude of the activation energies
All of these factors are involved.

Organic Chemistry
General organic chemistryWhat is a crystal? Recrystallization is a powerful technique for purification used ubiquitously in organic chemistry. It can allow a chemist to take an amorphous, impure lump of crude product and transform it into a pile of shiny, virtually contaminant-free crystals of the target sample. What are the best ways to grow pure, contaminant-free crystals? Describe the steps of recrystallization from dissolution to nucleation and finally to growth. What is the visual difference between a crystalline, pure sample and an impure crystal? What other physical properties differ between pure and impure solid samples? Write a short essay describing how recrystallization is used in the lab, how best to execute a recrystallization and answering these questions. Please cite and include all sources for your information, including pages from your lab manual/textbook.


Organic Chemistry
General organic chemistryWhen do we use Simple Past tense? Check ALL correct answers.
A) a feeling in the past
B) an action or event that was in progress
C) an action or event that was finished in the past
D) an action that started in the past and still continues
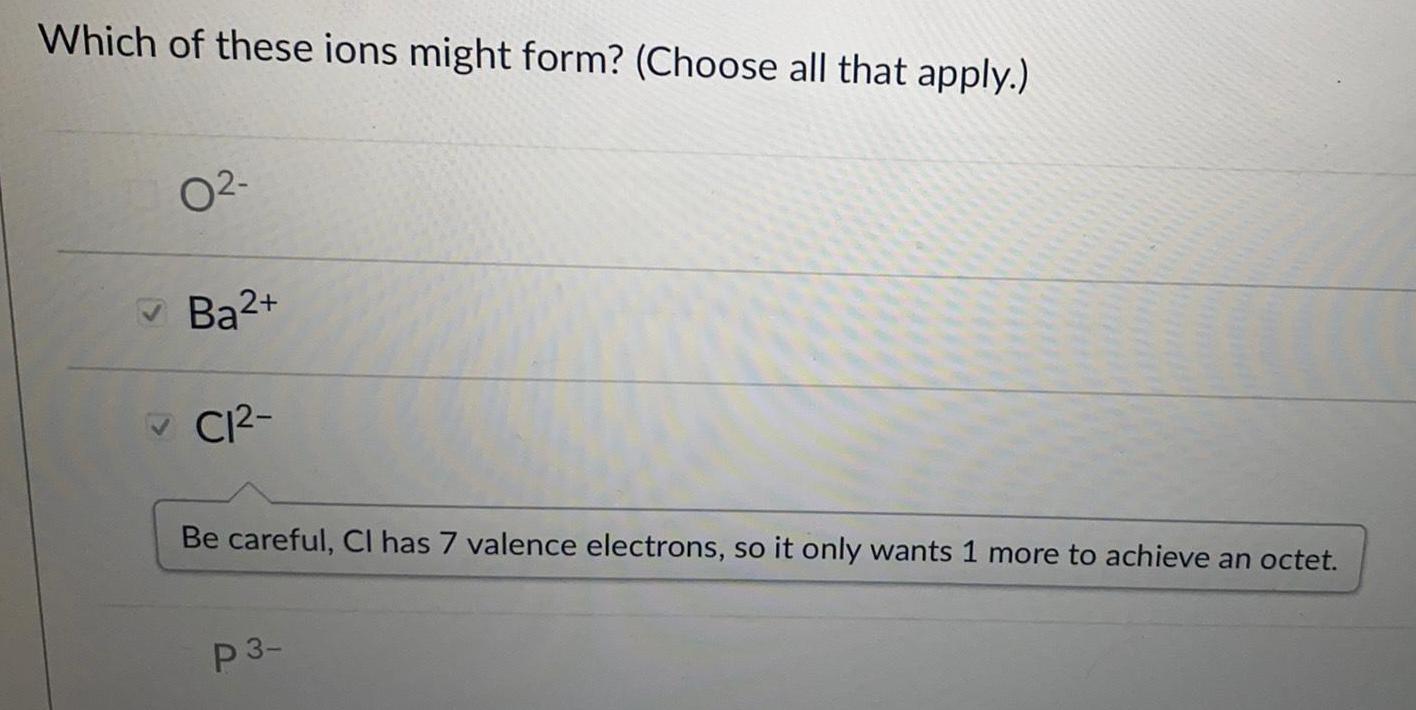

Organic Chemistry
General organic chemistryCheck the sentence with the CORRECT use of a stative verb.
A) Are you wanting to have more children now?
B) Do you want to have more children now?
C) Are you want to have more children now?

Organic Chemistry
General organic chemistrya. Convert phosphoenolpyruvate, an intermediate in glucose metabolism, to a skeletal structure.
b. Draw all reasonable resonance structures that have a phosphorus atom surrounded by 10 electrons.
c. Draw all reasonable resonance structures in which all second- and third-row atoms have an octet.

Organic Chemistry
General organic chemistryWhat does the following sentence mean (look at the main verb)?
"In recent years, Thayer has been talking to groups around the world."
A) A few years ago, she talked to other travelers.
B) She is talking to groups now.
C) She started talking to groups not a long time ago, and she still does it.
D) She does not talk to groups anymore.

Organic Chemistry
General organic chemistryWhat is true about the following sentence (look at the main verb)?
"They walked 1600 miles in intense heat across the Gobi Desert."
A) The events started in the past and continue in to the present
B) The events started and finished in the past.
C) The events are happening now.
D) The sentence expresses a habit or ritual.

Organic Chemistry
General organic chemistryWhat is the meaning of the following sentence?
"Since childhood, she has traveled widely in harsh climates and across rough lands."
A) She started traveling a lot when she was young, and she still does it.
B) She traveled a lot when she was a child.
C) She is traveling right now.
D) She travels a lot.

Organic Chemistry
General organic chemistryWhen do we use Past Progressive tense? Check ALL correct answers
A) For completed action or event
B) For an action or event that continued at some time in the past
C) For an action or event that continued when another action happened.
D) For a action or event happening currently.

Organic Chemistry
General organic chemistryWhen iron rusts in moist air, the product is typically a mixture of two iron-oxygen compounds. In one compound, there is an equal number of iron and oxygen atoms. In the other compound, there are three oxygen atoms for every two iron atoms. Choose the formulas for the two iron oxides. (Select all that apply.)
FeO
FeO₂
Fe₂O
Fe₂O 4
Fe₂O3

Organic Chemistry
General organic chemistryBalance the equation for the reaction of aluminum metal with solid iodine to form solid aluminum
iodide.
2Al(s) + 312(s) → 2A113(s)
Al2(s) + 312(s) → 2A113(s)
Al2(s) + 16(s) → 2A1I3(s)
Al(s) + I₂(s)→ All₂(s)
Al(s) + I(s)→ All(s)

Organic Chemistry
General organic chemistryClassify the following as a heterogeneous mixture, homogeneous mixture (solution), or a pure substance: hot tea with lemon juice
A. heterogeneous mixture
B. homogeneous mixture/solution
C. pure substance

Organic Chemistry
General organic chemistryDraw a structure of each of the following:
a. Aldehyde
b. Ketone
c. Ester
d. Carboxylic acid
e. Amide
f. anhydride
Your structure must contain at least 6 carbons and two substituents. Provide the IUPAC for each of your drawings

Organic Chemistry
General organic chemistryUse Avogadro's number (6.02 x 1023) to calculate how many hydrogen atoms are contained in exactly 1 teaspoon of water. The density of water is 1.00 g/mL and 1 teaspoon = 4.929 mL.
Hint: The molecular weight of water is 18.0 g/mol
2.97 x 1024 atoms
1.65 x 1023 atoms
1.22 x 1023 atoms
3.30 x 1023 atoms

Organic Chemistry
General organic chemistryEnter both the numeric valueland the units. Round the numeric value to three decimal places if necessary. Write the unit in the proper metric system abbreviated form.

Organic Chemistry
General organic chemistryWrite the composition of one atom of the 117Sn isotope.
protons
neutrons
electrons

Organic Chemistry
General organic chemistryThe mass number of an atom can be determined if the components of the atom are known.
What is the mass number of an atom containing 35 protons, 35 electrons, and 44 neutrons?
Mass number =

Organic Chemistry
General organic chemistryA beaker containing a mixture of water and ethanol needs to be separated into its components. These liquids can be separated by__ based on the liquids'__ properties.
chromatography, chemical
distillation; physical
reverse osmosis; physical
centrifugation; chemical

Organic Chemistry
General organic chemistry3- Write Henderson-Hasselbalch equation and define each term in this equation. Define the pH of an acidic solution and for a basic solution (give ranges). For a weak acid solution define the pka-value. What is the pka-values for formic acid, butanoic acid and acetic acid (all found in the biological environment) which acid is more acidic? Why? Draw the structure of these acids.

Organic Chemistry
General organic chemistryWhat is the atomic weight for the element that is in group 8A and period 1?
What is the atomic weight for the element that is in group 6A and period 2?
What is the atomic weight for the element that is in group 1B and period 5?
What is the atomic weight for the element that is in group 1A and period 6?

Organic Chemistry
General organic chemistryThe compound Cu(NH3)4SO4 is made the following reaction of copper II sulfate and ammonia:
CuSO4 (aq) + 4 NH3 (aq) → Cu(NH3)4SO4
a) If 10.0 g of CuSO4 are reacted, determine the theoretical yield of Cu(NH3)4SO4. b) The reaction is run to completion and you obtain 12.6 g of Cu(NH3)4SO4. What is the % yield of the reaction?

Organic Chemistry
General organic chemistryDraw the skeletal structure for the (CH3)2CHCOCH₂CH(CI)CH(OH)C(CH3)3

Organic Chemistry
General organic chemistryDecide if the following statement is true or false:
Protons and electrons are equal in mass, but opposite in charge.

Organic Chemistry
General organic chemistryThe element indium has two stable isotopes, indium-113 with an atomic mass of 112.9043 amu and indium-115 with an atomic mass of 114.9041 amu. From the atomic weight of In=114.82 one can conclude that:
indium-115 has the highest percent natural abundance
indium-113 has the highest percent natural abundance
both isotopes have the same percent natural abundance

Organic Chemistry
General organic chemistryWhat is the formula of the compound in which the atom combining ratios are:
chlorine : oxygen : fluorine = 1:3:1
Enter elements in the order given:

Organic Chemistry
General organic chemistry1 PCl5 + 4H₂O → 1 H3 PO4 + 5 HCl
If a reaction uses 12 moles of water, how much phosphoric acid (H3 PO4) will be produced?
4 moles
3 moles
48 moles
1 mole

Organic Chemistry
General organic chemistryA gas cylinder with a volume of 10.0 L contains 37.9 g of gas. What is the density of the gas?

Organic Chemistry
General organic chemistryA copper atom has a mass of 1.06 x 10 g and a penny has a mass of 2.5 g. Use this information to answer the questions below. Be sure your answers have the correct number of significant digits. What is the mass of 1 mole of copper atoms?

Organic Chemistry
General organic chemistryClassify each of the following mixtures as either homogeneous or heterogeneous:
oil and vinegar salad dressing
gasoline
sand and salt

Organic Chemistry
General organic chemistrya. Explain how the ionization energy of atoms changes when proceeding down a group of the Periodic Table
at first increases and then decreases
generally decreases
generally increases
at first decreases and then increases
b. Explain why this change occurs.
Electrons experience an increasingly stronger pull by the nucleus.
The nuclear charge is higher.
The shielding of an atom's outermost electrons decreases.
Electrons are farther from the positively charged nucleus..

Organic Chemistry
General organic chemistryAn organic solvent has a density of 1.18 g/mL. What volume is occupied by 30.0 g of the liquid?