Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
HydrocarbonsWhich 4 step process would convert the reactant to the product Excess reagent is implied if needed Hint Attempt this question towards the end of the exam so flag it if you get it in the beginning 1 Br dilute sulfuric acid 2 HBr t BUOK 3 HBr ROOR 1 03 2 water Me in NH3 1 4 H2 Lindlar NaOEt

Organic Chemistry
General organic chemistryHow many carbon atoms are bonded to the circled atom below Answer OH

Organic Chemistry
General organic chemistryCalculate the Keq if all are gases 3 H 0 0 600 atm 2 NH heat 1 000 atm 0 600 atm 0 400 atm First put in the chemicals then put in the numbers Pay close attention to the coefficients 1 N start 0 equil 0 200 atm Keq Keq a Nha b N C H d 1 e 2 3 g833 1 h 0500 10 400 27 X 0 300

Organic Chemistry
General organic chemistryWhat is the bond angle around the circled carbon atom below Select one O 90 O 270 O 109 5 O 120 180 OH

Organic Chemistry
General organic chemistryWhich of the following compounds would be expected to be the major product of the eaction below Br elect one O NaO Bu

Organic Chemistry
Practical DetectionMultiple Choice One of the most effective strategies families have in preparing for a potential terrorist threat is to create a plan and prepare a room or portion of a secure place in the home where they can shelter in place which is stocked with enough food water and supplies to last a minimum of five to seven days False O True

Organic Chemistry
Carboxylic acidsSpecify a synthetic scheme that would produce the compound shown above in the fewest steps possible Use one of the starting materials shown together with any of the available reagents Give the number of the starting material followed by the letters of the reagents in the order of their use for example 3be Starting Materials OCCH COC H Available Reagents a CH I b CH CH I e 1 OH 9 CHOCCHICH 2 CH Br Br Br Ilocos h Br 1 NaOC H Br 1 H 0 heat or NaOH H O then H O heat

Organic Chemistry
General organic chemistryPredict the products for each of the following D2 Pd D Lindlar

Organic Chemistry
Carboxylic acidsIdentify the reagents you would use to perform the following transformation Identify the reagents you would use to perform the following transformation CN The transformation above can be performed with some reagent or combination of the reagents listed below Give the necessary reagents in the correct order as a string of letters without spaces or punctuation such as EBF If there is more than one correct solution provide just one answer A PBr3 D excess NH3 B Na Cr O7 H SO4 H O E SOCI2 C NaCN F H3O heat

Organic Chemistry
General organic chemistryConvert the following line angle formula to a condensed structural formula t Draw the molecule on the canvas by choosing buttons from the Tools for bonds Atoms and Advanced Template toolba NN H 1200 EXP H C N O S

Organic Chemistry
PolymersWhat is the geometry around the circled carbon atom below OH CI HN Select one O planar 2D O linear 1D tetrahedral 3D N

Organic Chemistry
General organic chemistryWhich of the following alkenes a c reacts faster with HI b c d all react at same rate

Organic Chemistry
Chemistry in Daily LifeWhat is the percent yield of zinc nitrate when 4 02 moles silver nitrate is reacted with excess zinc chloride and 99 0g of zinc nitrate is produced Molar Mass of AgNO3 169 87g mol Molar Mass of Zn NO3 2 189 36g mol ZnCl2 2AgNO3 2AgCl Zn NO3 2 28 9 57 9 52 0 26 0

Organic Chemistry
General organic chemistryWhat is the formal charge on chlorine in the compound CIF Select one O a 1 O b 2 O c 0 O d 1

Organic Chemistry
General organic chemistrySynthesis Pick either A OH or B andl punite BqH a reasonable synthisay VIGR OH Ho

Organic Chemistry
Alcohols and PhenolsWhich 2 step process would convert the reactant to the product Excess reagent is implied if needed Hint Attempt this question towards the end of the exam so flag it if you get it in the beginning t BUOK 1 TsCl py OH 1 excess NaNH2 2 water 2 OsO4 Na2SO3 conc sulfuric acid dilute sulfuric acid OH HBr ROOR NaOEt HBr

Organic Chemistry
General organic chemistryWhen the compound below is treated with a strong base which of the following compounds would be the expected major product Br Select one Ne

Organic Chemistry
Chemistry in Daily Life1 What are the 4 assumptions of the kinetic molecular theory of gases 2 Convert 1011 5Pa to atmospheres 3 Your cat weighs 32 0 pounds It has four paws each one with an area of 1 0 square inches What pressure does your cat put on you when standing in pounds per square inch 4 The ideal gas law gives us PV nRT Solve this for n 5 For a two state problem where there is an initial and final state P V n T P V n T which are both equal to R In the case of constant temperature and volume T T and V V Solve this equation for P in this case it will be a function of P n and n only 6 A gas at 512K and 701torr is heated to 709K What is the final pressure if moles and volume are constant 7 3 00mol of gas at 79 C and 100 123Pa of pressure would have what volume 8 A reaction is performed at constant temperature and volume 5 00moles of reactant gases are put into the container originally The pressure increases from 1 20atm to 1 91atm How many moles of gases are in the container at the end 9 Extra Credit 1 000 mol of CH3CH OH gas is combusted with 2 000 mol of O2 gas in a container of constant volume and temperature and the pressure increases from 1 500atm to 1 551atm What is the yield

Organic Chemistry
General organic chemistryHow many chiral carbon atoms does the compound below contain

Organic Chemistry
General organic chemistryList all possible values of the magnetic quantum number m for a 4x electron M 0 an S

Organic Chemistry
General organic chemistryWhat reaction mechanism would be used for this reaction It results in an epoxide but what reaction method would be used 2 Describe the reaction mechanism of the following reaction 2 Describe the reaction mechanism of the following reaction OH TsCl OH pyridine OH OTS NaOH C

Organic Chemistry
General organic chemistryExamine the radicals below closely Draw out and consider any resonance structures each might have I W III Which radical is the most stable Select Select Which radical is the least stable 1 DY II IV

Organic Chemistry
General organic chemistryA A main group element with the valence electron configuration 3s 3p is in periodic group It forms a monatomic ion with a charge of B A main group element with the valence electron configuration 2s22p is in periodic group It forms a monatomic ion with a charge of

Organic Chemistry
Alcohols and PhenolsWhich 3 step process would convert the reactant to the product Excess reagent is implied if needed Hint Attempt this question towards the end of the exam so flag it if you get it in the beginning OH 1 1 BH3 THF 2 H2O2 NaOH 1 Hg OAc 2 aq 2 NaBH4 Br2 2 x KMnO4 NaOH dilute sulfuric acid HBr ROOR OH 3 HBr t BUOK TsCl py

Organic Chemistry
General organic chemistryName the alkene below Hint make sure to include all needed stereo descriptors without using parentheses

Organic Chemistry
Practical DetectionFor a quick grade explain using complete sentences correct quantities and academic vocabulary How would I make 500 0 ml of a 0 25 M copper II sulfate solution using the equipment and chemicals on my desk Troy Day 6 35 AM 100 points Your answer Ass

Organic Chemistry
General organic chemistryQuestion 35 1 point Methotrexate image below is classified as an antimetabolite Methotrexate most closely resembles which endogenous compound NH M N avoi HO O5 hydroxytryptamine Aspartic acid Histamine Folate Epinephrine Next Page Page 3

Organic Chemistry
Practical DetectionThe mass spectrum of 3 pentanone has a very large ion peak at m z 57 Which of the following ions is thought to be responsible for this peak O A OB OC CH3CH CHCH3 CH3 3C A CH5C308 CH CH CH B C D
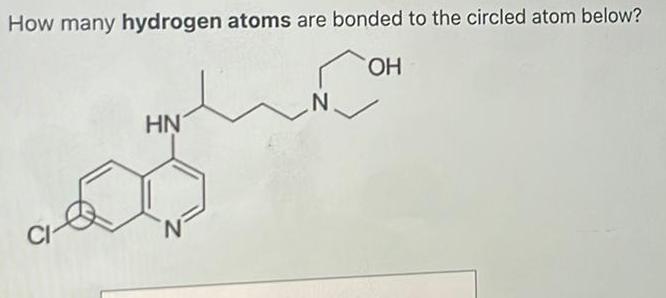
Organic Chemistry
General organic chemistryHow many hydrogen atoms are bonded to the circled atom below OH CI HN N N

Organic Chemistry
General organic chemistrygiven the major products for each of the following reactions or sequences of reaction show all relevant stereochemistry 4 Br 1 Mg ether 2 CO 3 H3O

Organic Chemistry
Biomolecules20 Draw a cis fatty aci 21 Which fatty acid in the figures below would you expect to be most soluble in water HO HO 22 Which fatty acid in the figures below will have a higher melting point HO Octanoic acid trans Oleic acid OH

Organic Chemistry
Practical DetectionFill in the nuclide symbol for the missing particle in the following nuclear equation e N

Organic Chemistry
Halogen DerivativesDraw eclipsed least stable conformation using Newman projection for the compound below H C H C A H CHCI H H C CI B H H H H H Me H H C H C CI C CI CHH H H C Ch D H H CHCH

Organic Chemistry
PolymersA Is CH O polar or nonpolar B Is NF3 polar or nonpolar C Is NH Cl polar or nonpolar ences to access in

Organic Chemistry
Practical DetectionWrite the dissolution reaction for cobalt II chloride in water Be sure to specify the state of each reactant and product Is cobalt II chloride considered soluble or not soluble A Soluble B Not soluble Based upon this the equilibrium constant for this reaction will be A Greater than 1

Organic Chemistry
HydrocarbonsOH Select one OEA O EA O EA O EA HCI H O CI reaction ordinate reaction ordinate reaction ordinate

Organic Chemistry
General organic chemistryThe enthalpy change for the following reaction is 121 kJ Using bond energies estimate the C H bond energy in CH4 g CH4 g Cl g CH3CI g HCl g kJ mol

Organic Chemistry
General organic chemistryMatch the reagents with each step Drag and drop options on the right hand side to reorder and match with items on the left Reordering may cause items on the right hand side to swap positions Step 1 Step 2 Step 3 Step 4 Step 5 CH3CH Li THF H O NaNHz NH3 epoxyethane CH 2 CH3CH Br III III

Organic Chemistry
Chemistry in Daily LifeWhat best explains why the formation of ionic solids is exothermic Select one O a The formation of an ionic solid from gaseous cations and anions releases ene O b Chemical reactions always release energy O c Metals want to lose electrons and nonmetals want to gain electrons O d None the formation of ionic solids is not usually exothermic

Organic Chemistry
Aldehydes & KetonesBr H Draw the curved arrow mechanism for the reaction between the aldehyde and propyl bromide including the final product Be sure to include nonzero formal charges and lone pair electrons on all appropriate atoms Draw the resonance structure of the enolate ion only Do NOT include curved arrows in this box need help with 3rd box only thx


Organic Chemistry
General organic chemistryBr Draw the curved arrow mechanism for the reaction between the aldehyde and propyl bromide including the final product Be sure to include nonzero formal charges and lone pair electrons on all appropriate atoms Draw the resonance structure of the enolate ion only Do NOT include curved arrows in this box need help with 3rd box only thx

Organic Chemistry
HydrocarbonsThe numbered elementary steps below each belongs each step with its type I E IV Br Br heat Br Br Choose Choose propagation step Br I HBr

Organic Chemistry
General organic chemistryWhat is the molar mass of a gas if 1 15 g of the gas nas STP Your answer should have three significant figures Round to the nearest whole number


Organic Chemistry
Reactions of benzene10 2 Draw resonance structures for each of the following radicals the a wwwwwww b c d

Organic Chemistry
General organic chemistryWhich one of the following is the general form of the Lewis dot formula for chlorine X

Organic Chemistry
PolymersA Is XeO4 polar or nonpolar B Is SCI polar or nonpolar C Is SO polar or nonpolar V

Organic Chemistry
Practical Detection4 attempts left Check my work Be sure to answer all parts MTBE CH 20 is a high octane gasoline additive with a sweet nauseating odor Because small amounts of MTBE have contaminated the drinking water in some towns it is now banned as a fuel additive in many areas MTBE reacts with O to form CO and H O Write a balanced equation for the combustion of MTBE Do not included states of matter in your answer So Guided

Organic Chemistry
HydrocarbonsMatch the following items Drag and drop options on the right hand side to reorder and match with items on the left Reordering may cause items on the right hand side to swap positions Step 1 Step 2 Step 3 CH3CH CH CH Br Na NH3 NaNH2 NH3 2 equivalents Cl 2 equivalents HBr