Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
General organic chemistryUsing the periodic table, what do you know about Rubidium?
It has a high electronegativity and a high reactivity
It is a small atom and has a low electronegativity
It has a low electronegativity and has a high reactivity
It has a low electronegativity and has a low reactivity
It has a high electronegativity and a low reactivity

Organic Chemistry
Chemistry in Daily LifeA person fully reacts 0.75mol of magnesium metal with excess hydrochloric acid (HCI) according to the reaction below. If the products are at a pressure of 1.5atm and a temperature of 50°C, what volume of hydrogen gas was formed?
(R=0.0821)
Mg(s) + 2HCl(aq) --> MgCl2(aq) + H2(g)
123.2L
9.5L
19.9L
13.3L
0.0754L
Organic Chemistry
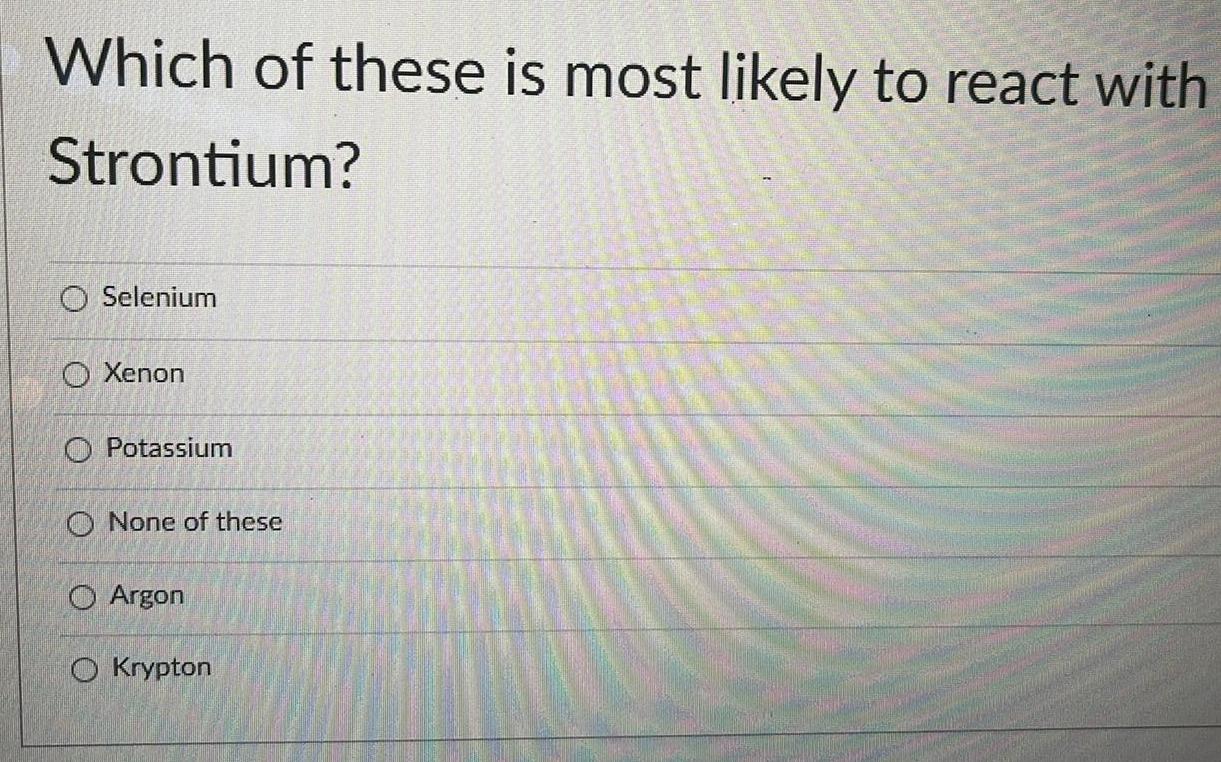
General organic chemistryWhich of these is most likely to react with
Strontium?
Selenium
Xenon
Potassium
None of these
Argon
Krypton

Organic Chemistry
Chemistry in Daily LifeExplain the following trends in acidity same as yo
did in question 2
FCH₂COOH > CICH₂COOH > CH3COOH>CH3CH₂OH

Organic Chemistry
General organic chemistryHow will an LOL diagram show energy being conserved?
Energy will be destroyed.
There will be more energy in the reactants than products
All energy will be accounted for
There will be more energy in the products than in the reactants
The energy won't change

Organic Chemistry
General organic chemistryWhat is true about nuclear fission?
Two small nuclei come together.
It is not used in atomic bombs.
It is occuring in the sun.
Scientists have not yet been able to harness nuclear fission to create electricity.
It occurs when a large unstable nucleus splits into smaller nuclei.

Organic Chemistry
General organic chemistryThe effective nuclear charge, Zeff, for a valence electron can be approximated using the core charge of the atom; that is, the net charge of the nucleus and the inner (nonvalence) electrons. Determine the core charge for an atom of Ar.

Organic Chemistry
Practical DetectionA person has 100mL of a 1M NaCl Solution. If the solution was diluted to 0.25M with water, what will the final volume of the solution be?
0.125L
50mL
200mL
300mL
400mL

Organic Chemistry
Practical DetectionVolume of the starting solution:
Cyclohexane Component
Vapor temperature when distillation of cyclohexane started:
Vapor temperature when distillation of cyclohexane finished:
Volume of cyclohexane collected:
Density of cyclohexane collected:
Mass of cyclohexane collected (g)
30.0 mL
80.0 °C
83.0 °C
20.1 mL
0.779 g/mL

Organic Chemistry
Practical DetectionZinc metal and sulfuric acid (H₂SO4) react to form zinc (II) sulfate and hydrogen gas. What are the chemical formulas for the products of the reaction?
ZnSO4 + H₂
Zn(SO4)2 + S
ZnSO4 + H
H₂SO4 + Zn
ZnS + H₂

Organic Chemistry
General organic chemistryIf 24g of hydrogen gas is fully reacted with excess carbon, how many particles, in moles of benzene
(C6H6) will form?
6C(s) + 3H2(g)--C6H6
2.3mol Benzene
4mol Benzene
24mol Benzene
312g Benzene
12mol Benzene

Organic Chemistry
General organic chemistrySulfur has how many valence electrons? What is it's charge when it is stable?
2,6
2,2
6, -6
6,6
6,2
6, -2

Organic Chemistry
Chemistry in Daily LifeWrite the curved arrow mechanism for the protonation of imidazole. Use H* (the proton) as the acid. Look at the "availability" of the lone pairs and resonance after protonation (resonance stabilization), to decide which one of the two nitrogens to protonate.

Organic Chemistry
General organic chemistryUse the pka table to select a base to deprotonate nitrous acid (HNO₂) pka = 3.35 Write the acid base reaction Calculate the equilibrium constant to demonstrate that you selected a good base. Write the curved arrow mechanism in both directions

Organic Chemistry
General organic chemistryWrite the curved arrow mechanism for the dissociation of sulfuric acid in water (show all bonds and lone pairs, i.e. Lewis structures) Which will be more abundant at 25 °C, sulfuric acid or hydronium ion? Why? What type of acid base reaction is this? Define the role of water, i.e Lewis base, Brønsted-Lowry acid, etc.

Organic Chemistry
General organic chemistryIf 109g of water was created in the following equation, how much energy was released?
CH4(g)+202(g) CO2(g) +2H₂O) AH = -890.8
Round to two decimal places.

Organic Chemistry
General organic chemistryWhich of these is the least electronegative?
Sodium
Rubidium
Potassium
Hydrogen
Lithium

Organic Chemistry
General organic chemistryIf a person has 621mL of a 4.2M KCI solution. How many moles of KCI would be in the solution? (1000mL-1L) Round to two decimal places.

Organic Chemistry
Practical DetectionA person places 8.2mol of sodium chloride in a beaker, and they fill the beaker with water until the 89mL mark and stir. What is the concentration (molarity) of the solution they
0.092M
92.1M
9.2M
0.011M
10.9M

Organic Chemistry
Practical DetectionA person has a 2L sample of gas with a pressure of 2atm, a temperature of 25°C. How many particles, in moles, does the person have?
(R=0.0821)
0.016mol
0.16mol
2.42mol
6.12mol
100mol

Organic Chemistry
Practical DetectionA person combines 4g of A with some reactant B to form 25g of C and 8g of D according to the reaction below. Assuming the reactants were completely used up, what mass of reactant B was reacted? A+B-->C+D

Organic Chemistry
General organic chemistryWhat is true about an endothermic reaction?
They often feel hot to the touch.
More bonds are being broken than formed
More bonds are being formed than broken
Equal amounts of bonds are being broken and formed
No bonds are being broken or formed

Organic Chemistry
Practical DetectionA scientist expects to get 5.9g of sodium chloride based on their calculations. In the lab, they collect 5.5g of sodium chloride. What is the percent yield for their reaction?
2Na(s) + Cl2(g) --> 2NaCl(s)
107%
93.2%
72%
0.9%
89%

Organic Chemistry
General organic chemistryIf 3mol of solid carbon is reacted with 3mol of hydrogen gas, what mass of benzene (C6H6) is likely to form?
6C(s) + 3H2(g) --> C6H6(1)
234g Benzene
0.5g Benzene
78g Benzene
39g Benzene
24.2g Benzene

Organic Chemistry
General organic chemistryIf a person has 162mol of K₂O, what mass, in grams, of K₂O doo they have? Round to two decimal places.

Organic Chemistry
Chemistry in Daily LifeThe FHA/VA Loan Addendum is used if
(a) The seller needs to inform buyers of multiple offers.
(b) The buyer is going to apply for an FHA or VA loan.
(c) The buyer wants an inspection or assessment.
(d) The renter wants to purchase the property.

Organic Chemistry
General organic chemistryThe molarity (M) of the Ca(NO3)2 solution when 61.3 mL react with 46.2 mL of 5.2 M Na3PO4

Organic Chemistry
General organic chemistryHow would you prepare the following two compounds starting from benzene? (Proper sequence of steps with proper reagents necessary for each step must be given)
HO.
-NH₂
HO-
-NH₂

Organic Chemistry
General organic chemistryYou have 125.0 mL of a solution of H3PO4, but you don't know its concentration. If you titrate the solution with a 4.56 M solution of NaOH and reach the endpoint when 134.1 mL of the base are added, what is the concentration of the acid?
![Calculate the K, for the following hypothetical reaction, using the given equilibrium
concentrations: [A] = 0.020 M, [B] = 0.030 M and [C]=7.74 M
A (g) + 2B (g) = C()
a. 4.3 x10³
b. 1.3x10*
c. 7.7 x10³
d. 2.3x10€](https://media.kunduz.com/media/sug-question/raw/80376686-1659894415.6000364.jpeg?w=256)
Organic Chemistry
Practical DetectionCalculate the K, for the following hypothetical reaction, using the given equilibrium
concentrations: [A] = 0.020 M, [B] = 0.030 M and [C]=7.74 M
A (g) + 2B (g) = C()
a. 4.3 x10³
b. 1.3x10*
c. 7.7 x10³
d. 2.3x10€
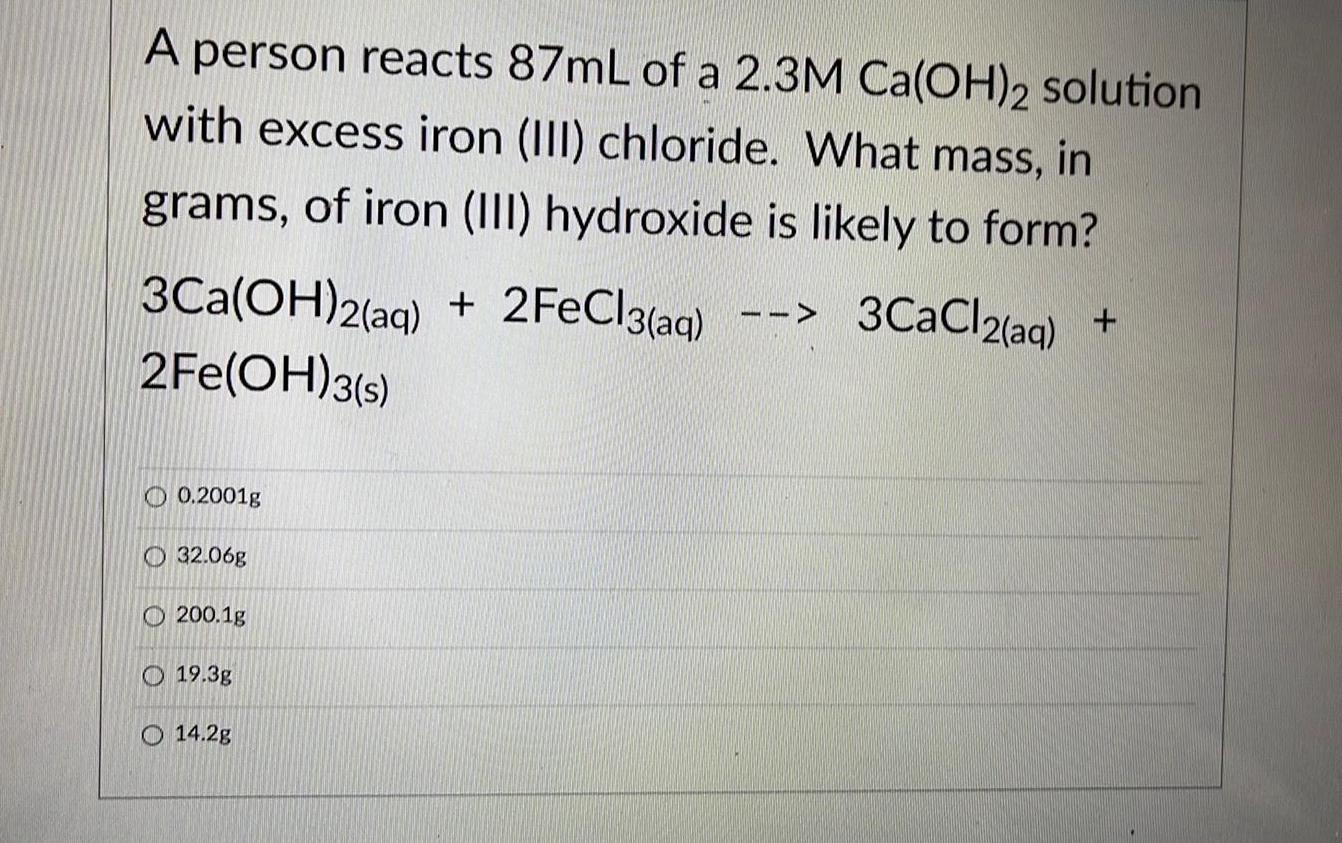
Organic Chemistry
Practical DetectionA person reacts 87mL of a 2.3M Ca(OH)2 solution with excess iron (III) chloride. What mass, in
grams, of iron (III) hydroxide is likely to form?
3Ca(OH)2(aq) + 2FeCl3(aq) --> 3CaCl2(aq) +2Fe(OH)3(s)
0.2001g
32.06g
200.1g
19.3g
14.2g

Organic Chemistry
Practical DetectionA person creates a 0.5L solution using 164.25g of solid HCI. What will the concentration (molarity) of the solution be?
328.5M
9M
15.6M
4.5M
10.25M

Organic Chemistry
PolymersA person dissolves 120g of NaCl in 100mL of water. If the person dilutes their solution by adding another 300mL of water, what mass, in grams, of sodium chloride (NaCl) will be in the new solution?
12g
75.2g
120g
1200g
98.6g

Organic Chemistry
Practical DetectionWhat type of reaction is below?
Mg(s) + 2HCl(aq) --> MgCl2(aq) + H2(g)
Synthesis
Combustion
Decomposition
Double Replacement
Single Replacement

Organic Chemistry
General organic chemistryWhich of the following solutions could be used to titrate a HCl solution with an unknown concentration if the titration was using a pH indicator?
1.5M HBr solution
2M NaCl solution
5.2M LICI solution
2M NaOH solution
1.68M water solution

Organic Chemistry
General organic chemistryWhy does a balanced equation represent mass
being conserved?
Balancing an equation results in different amounts of mass in the reactants and products
Balancing a reaction creates mass.
Balancing a reaction results in the same molecules being present in the reactants and products.
Balancing a reaction results in the same number and type of atom being present in the reactants and products.
Balancing a reaction changes the type of substance being reacted, so mass is neither created nor destroyed.

Organic Chemistry
General organic chemistryWhat is the chemical formula for Aluminum Bisulfate?
AIBI3
Al2(SO4)3
AI(HSO4)3
AI(HSO4)2
OAIHSO4

Organic Chemistry
HydrocarbonsWhat coefficients would result in the below chemical equation
being balanced? (The coefficients in the answers are written in the
same order as they would be placed into the chemical equation.)
Chemical Equation:
_Al(s) + HCl(aq) --> ___AICI3(s) +___H2(g)
4,16,4,6
2,6,3,2
2,6,2,3
1,3,1,1
O2,3,1,1

Organic Chemistry
IsomerismWhat would the products of a reaction between aqueous copper (II) chloride and aluminum metal be?
Cu +AICI
CuCl2+Al
AICI₂ + Cu
CuAl + Cl₂
Cu + AICI 3

Organic Chemistry
General organic chemistryAccording to IUPAC, when counting the carbons in a branched hydrocarbon chain you would use the shortest possible chain.
True
False

Organic Chemistry
Alcohols and PhenolsTrue / False: An alcohol is an organic compound that contains the hydroxyl group
True
False

Organic Chemistry
Chemistry in Daily LifeIn a chemical reaction that takes place at a fixed pressure and volume in a calorimeter, the enthalpy change (AH) is 585 kJ/mol. Will this reaction result
in an increase or a decrease in the calorimeter's water temperature?
There is not enough information to know if the temperature will increase or decrease.
There will be a decrease in temperature.
There will be no change in temperature.
There will be an increase in temperature.

Organic Chemistry
Chemistry in Daily LifeIron metal reacts with a copper sulfate solution, CuSO4(aq) Which of the following would result in the lowest rate of reaction?
A solid piece of iron in 1 M CuSO4(aq)
Iron powder in 1 M CuSO4(aq)
Iron powder in 2 M CuSO4(aq)
A solid piece of iron in 2 M CuSO4(aq)

Organic Chemistry
Practical DetectionWhich of the following statements is most accurate?
Energy is stored in chemical bonds and released when the bonds are broken.
Energy is absorbed when chemical bonds are broken and released when new bonds form.
More energy is required to form a chemical bond than the energy required to break a chemical bond.
Answers A and C are both true.

Organic Chemistry
HydrocarbonsM/ Ch. Hydrohalogenation involves the reaction of an alkene with which of the following?
hydrogen
a hydrogen halide
a halogen
water

Organic Chemistry
Practical DetectionWhat are the original and final oxidation numbers for iron in the smelting of iron
from iron oxide?
Fe₂O3(s) + 3CO(g) → 2Fe(s) + 3CO₂(g)
No Change
+3 --> 0
0 -- +2
+2 --> 0

Organic Chemistry
General organic chemistryWhich of the following statements does not characterize an oxidizing agent?
The oxidation number of an oxidizing agent decreases
An oxidizing agent gains electrons
An oxidizing agent causes another species to be oxidized
An example of a good oxidizing agent is an alkali metal, such as sodium (Na)

Organic Chemistry
Aldehydes & KetonesTrue False: The product of a hydrogenation reaction of an aldehyde or a ketone is an ester
True
False

Organic Chemistry
IsomerismTrue / False; When naming compounds you can only use the cis/trans naming system for alkyne molecules.
True
False

Organic Chemistry
General organic chemistryM/ Ch. Many alcohols are made by the addition reaction of what two types of compounds?
water and alkenes
alkanes and alkenes
Oesters and ethers
water and alkanes