Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
General organic chemistryBe sure to answer all parts.
Predict the geometry of the following species using the VSEPR model. Type the number corresponding
to the correct geometry in the box next to the formula.
(a) PCl3
(b) CHCl3
(c) SIH4
(d) TeCl4
Electron Domain
Molecular
1 - bent
2 - linear
3-octahedral
4 - seesaw-shaped
5-square planar
6-square pyramidal
7- tetrahedral
8- trigonal bipyramidal
9- trigonal planar
10 trigonal pyramidal
11 - T-shaped

Organic Chemistry
Practical DetectionThe following reaction is first order in
respect to NO and second order in
respect to O₂. With this in mind, what
is the rate constant for the reaction?
Be sure you choose the answer with
the right units.

Organic Chemistry
Chemistry in Daily LifeThe unbalanced equation below shows the
combustion of methane.
_CH4 + _O2 ---> _CO₂ + _H₂O
Which series of numbers represents the coefficients
necessary to balance the equation?
2, 2, 1, 4
0, 3, 2, 2
1, 1, 1, 2
1, 2, 1, 2

Organic Chemistry
General organic chemistryBe sure to answer all parts.
Tin (Sn) exists in Earth's crust as SnO₂. Calculate the percent composition by mass of Sn and O in SnO₂.
% Sn
% O

Organic Chemistry
General organic chemistryEthane and acetylene are two gaseous hydrocarbons. Chemical analysis show that one sample of ethane contains 2.65 g of carbon and 0.668 g of hydrogen, and one sample of acetylene contains 4.56 g of carbon and 0.382 g of hydrogen. Write reasonable empirical formulas for these compounds.
Ethane:
Acetylene:

Organic Chemistry
Chemistry in Daily LifeExamine each of these chemical equations to
determine which equation is correctly balanced.
Mg(NO3)₂ +K₂CO3 → MgCO3 + KNO3
Mg(NO3)2 +2K₂CO3 → MgCO3 + 2KNO3
2Mg(NO3)₂ +K₂CO3 → 2MgCO3 + KNO3
Mg(NO3)2 +K₂CO3 → MgCO3 + 2KNO3

Organic Chemistry
Chemistry in Daily LifeCalculate the pressure, in atmospheres, of 8.67 mol CO(g) in a 2.0 L tank at 48 degrees C.

Organic Chemistry
General organic chemistryWhat is the molarity of a solution prepared by dissolving 6.0 grams of NaOH (molecular mass = 40.0 g/mol) to a total volume of 300 ml.
0.5 M NaOH
133.3 M NaOH
20.0 M NaOH
2.0 M NaOH

Organic Chemistry
Practical DetectionDuring kinetic studies, the following reaction was determined to be second order in respect to NO and zeroth order in respect to O₂. What is the overall order for the reaction?
2 NO (g) + O₂ (g) ⇒ 2 NO₂ (g)
first order
third order
second order
zeroth order

Organic Chemistry
Carboxylic acidsCalculate the molarity of an acetic acid solution if 42.62 mL of the solution is needed to neutralize 102 mL of 1.56 M sodium hydroxide. The equation for the reaction is
HC2H3O2(aq) +NaOH(aq)⇒Na+(aq)+C2H302-(aq)+H20(aq)

Organic Chemistry
General organic chemistryBe sure to answer all parts.
Write chemical formulas for the following molecular compounds:
(a) nitrogen triiodide
(b) tetranitrogen decoxide
(c) xenon trioxide
(d) diiodine pentoxide

Organic Chemistry
General organic chemistryJoe checked his display in his car and saw that he traveled 97 km in 100 minutes. What is Joe's average speed in miles per hour?
The conversion factor you will need for this question is: 1 mile = 1.60934 km
miles per hour. (Round to the nearest tenth)

Organic Chemistry
Halogen DerivativesType the following formulas in the boxes below in order of increasing boiling point: RbF, CO2, CH3OH, CH₂Br.

Organic Chemistry
Reactions of benzeneOn the basis of the general mechanism for acid-catalyzed ester hydrolysis shown in the mechanism in class 16 key, draw an analogous sequence of steps for the specific case of ethyl benzoate hydrolysis.
Look up the structure of ethyl benzoate.

Organic Chemistry
Chemistry in Daily LifeWhy is John Maynard Keynes often considered the savior of liberalism between the two world wars?
a) He suggested a way for government to intervene in private economic matters, because he
recognized that businesses cannot save the economy.
b) He told people that investing in a central bank was a bad idea.
c) He reminded people that inequality can only be cured with individual effort.
d) He urged a greater role for business in a country's economic life.

Organic Chemistry
IsomerismA doctor orders a Liter of medication to be administered over the course of 8 hours. How should the IV drip be set in
drops per minute?
The conversion factor you will need for this question is: 1 mL equals 10 Drops
Which of the following could be next step in setting up dimensional analysis?
(1L/8 hours) (1,000 mL/1L) (?/?)
(1 hour/60 liters)
(60 minutes/1 hour)
(1 mL/10 drops)
(10 drops/1 mL)

Organic Chemistry
General organic chemistryWrite the abbreviated electron configurations for
the elements below.
Sulfur
Manganese

Organic Chemistry
General organic chemistryWhich functional group(s) would be added to 1 methylcyclohexene using the reagents below: Br2, H₂O
ketone and aldehyde
hydrogen
bromine and hydrogen
bromine and hydroxyl
ketone
hydrogen and hydroxyl
bromine
hydroxyl
aldehyde

Organic Chemistry
Practical DetectionHow many moles of Fe₂O3 are in 211 g of the compound?
number of moles:
![Provide a sequence of laboratory reactions which could be used to prepare 2-methylpropylmagnesium bromide [whose formula is (CH₂)2CHCH₂MgBr] starting with 2-methylpropene. Your sequence must proceed through an alcohol as an intermediate product.](https://media.kunduz.com/media/sug-question/raw/59428215-1659632575.5425308.jpeg?w=256)
Organic Chemistry
Alcohols and PhenolsProvide a sequence of laboratory reactions which could be used to prepare 2-methylpropylmagnesium bromide [whose formula is (CH₂)2CHCH₂MgBr] starting with 2-methylpropene. Your sequence must proceed through an alcohol as an intermediate product.

Organic Chemistry
Practical DetectionDimensional Analysis 1: Unit Conversions:Question 8
Convert 80 hours into seconds (60 seconds = 1
minute; 60 minutes = 1 hour). Round to two decimal
places when necessary (5.05).
Enter answer below
Enter your response

Organic Chemistry
Chemistry in Daily LifeEach step in the following process has a yield of 70.0%.
CH4 +4Cl₂→ CCI4 + 4HCI
CCI + 2 HF→ CCI₂F₂ + 2HCl
The CCI, formed in the first step is used as a reactant in the second step.
If 8.00 mol CH₂ reacts, what is the total amount of HCI produced? Assume that Cl, and HF are present in excess.
moles HCl: mol

Organic Chemistry
Practical DetectionAllyl sulfide, C6H₁0S, in the substance that gives garlic, onion and leeks their characteristic odor. Some studies indicate that garlic may beneficial for the heart
and in lowering cholesterol.
a. How many moles of H are in 0.75 moles of C6H10S?
b. How many moles of S are in 23.2 g of C6H10S?
c. How many grams of C are in 44.0 g of C6H10S?

Organic Chemistry
IsomerismIn a cycle of copper experiment, a student first reacts a piece of copper metal with nitric acid to produce copper(II) nitrate
(Cu(NO3)₂) solution. The student then performs various reactions which transform the copper ions into a series of different copper compounds and complexes. Finally, the last reaction reduces the copper ions back to elemental copper metal. Copper atoms are conserved throughout the process.
If the initial step of the experiment produces 5.14 mL of 1.66 M Cu(NO3)2, what is the theoretical yield of solid copper (Cu) that can be recovered at the end of the experiment?

Organic Chemistry
BiomoleculesWhat amino acid sequence does the following mRNA nucleotide sequence specify?
5' -AUGAACCUAUGC-3'
Express the sequence of amino acids using the three-letter abbreviations, separated by hyphens (e.g., Met-Ser-T
Gly).

Organic Chemistry
General organic chemistryDimensional Analysis 1: Unit Conversions:Question 1
Complete the conversion indicated in the image. 7meters to inches
(1 meter = 39.4 inches). Round to two decimal
places when necessary (5.05).

Organic Chemistry
BiomoleculesSort the following 10 codons into one of the three bins, according to whether they code for a start codon, an in-sequence a or a stop codon.

Organic Chemistry
Chemistry in Daily LifeA piece of Zn was added to CuSO4solution. Initially CuSO4 (aq) is a blue/green color and after 30 minutes it turned light blue. Initially the Zn (s) was dull gray and after 30 minutes it was black.
Based on the above observations, answer the following questions:
a. What evidence is there of a chemical reaction?
b. Write a balanced equation for this reaction.
c. What type of chemical reaction is this?

Organic Chemistry
Chemistry in Daily LifeInitially magnesium is a shiny light gray ribbon of material. When placed in flame heat and light energy are produced and a white ash is produced.
Based on the above observations, answer the following questions:
a. What evidence is there of a chemical reaction?
b. Write a balanced equation for this reaction.
c. What type of chemical reaction is this?
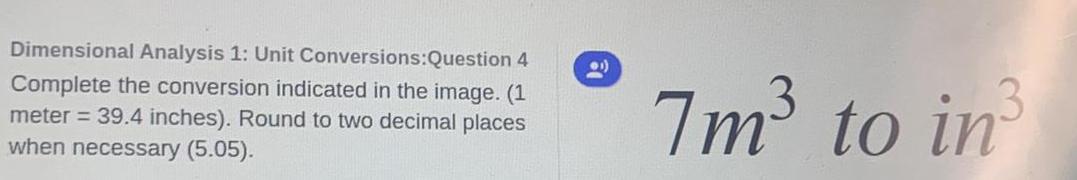
Organic Chemistry
Chemistry in Daily LifeDimensional Analysis 1: Unit Conversions:Question 4
Complete the conversion indicated in the image. (1
meter = 39.4 inches). Round to two decimal places
when necessary (5.05).
1)
7m³ to in³

Organic Chemistry
General organic chemistryDraw the unique stereoisomers for 2-chloro-2,3-dimethylpentane. Show stereochemistry clearly. To ensure proper grading, explicitly draw all four groups, including wedge/dash bonds, around a chirality center. Indicate whether the compounds could exist in an optically active form.

Organic Chemistry
General organic chemistryJailah needs a replacement for lithium for a science experiment. She tries silicon, but it does not work. Using what you know about patterns in the periodic table, which advice would you give Jailah?
A. Try a lanthanide series element.
B. Try a non-metal from the halogen group.
C. Try an element in the same group as lithium.
D. Try an element in the same period as lithium.

Organic Chemistry
General organic chemistryBlast furnaces extra pure iron from the iron(III) oxide in iron ore in a two step sequence. In the first step, carbon and oxygen react to form carbon monoxide:
2C(s) + 02(8)⇒2 CO (g)
In the second step, iron(III) oxide and carbon monoxide react to form iron and carbon dioxide:
Fe₂O3(s) + 3CO(g)⇒ 2Fe(s) + 3 CO₂ (g)
Suppose the yield of the first step is 72.% and the yield of the second step is 91.%. Calculate the mass of oxygen required to make 10.0 kg of iron.
Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.

Organic Chemistry
General organic chemistryPure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3.82 g of magnesium ribbon burns with 7.80 g of oxygen, a bright, white light and a white, powdery product are formed.
Enter the balanced chemical equation for this reaction. Be sure to include all physical states.
equation:
What is the limiting reactant?
magnesium
oxygen
If the percent yield for the reaction is 80.1%, how many grams of product were formed?

Organic Chemistry
Chemistry in Daily LifeIn which of these substances are the atoms held together by polar covalent bonding?
Multiple Choice
SrCl2
CsCI
CIF
TIF2
S8

Organic Chemistry
General organic chemistryAqueous hydrochloric acid (HCI) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H₂O). What is the theoretical yield of water formed from the reaction of 0.36 g of hydrochloric acid and 0.24 g of sodium hydroxide? Round your answer to 2 significant figures.

Organic Chemistry
General organic chemistryConsider a compound with the fictional element Tiktokium (Tk). The element Tk has 6 valence electrons and,
like most elements, prefers an octet of 8 electrons around it.
CTKBr2 (carbon is central)
Draw the Lewis structure and answer the following questions:
a. What is the total valence electron count for this compound?
b. How many double bonds are there?
c. How many single bonds are there?
d. How many lone pairs are there total?
e. How many lone pairs are around the Tk atom?
f. How many lone pairs are around the Catom?

Organic Chemistry
Chemistry in Daily LifeWhich of the following atoms contains only three
valence electrons?
Li
F
B
N
Ne

Organic Chemistry
General organic chemistryWhat is the symbol for the atom that has the
electron configuration 1s²2s²2p63s²3p64s²3d7?

Organic Chemistry
Chemistry in Daily LifeHow many orbitals are in the d sublevel?
How many electrons can the d-orbitals hold?
How many orbitals are in the f sublevel?
How many electrons can the f-orbitals hold?

Organic Chemistry
Practical DetectionA 1.5% KBr (w/v) solution in water is produced by mixing...
1.5 moles of KBr in 100 moles of water.
1.5 moles of KBr with 100 ml of water.
1.5 g of KBr in a solution of 100 ml.
1.5 g of KBr with 100 ml of water.

Organic Chemistry
Halogen DerivativesWhich is NOT part of the Kinetic Molecular theory?
Pressure of a gas is a measure of the number of hits the gas molecules have on
its container.
At the same temperature, smaller particles move more quickly that larger
particles.
The motion of particles increases as the surrounding temperature increases.
The motion of particles decreases as the surrounding temperature increases

Organic Chemistry
General organic chemistryThe Lewis dot symbol consists of the symbol for the element surrounded by dot(s). What does the dot or dots represent?
Multiple Choice
electron configuration
valence electrons
atomic number
atomic mass
core electrons

Organic Chemistry
General organic chemistryLiquid octane (CH3 (CH₂) CH3) will react with gaseous oxygen (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). Suppose 68.5 g of octane is mixed with 84. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to 2 significant digits.

Organic Chemistry
Chemistry in Daily LifeWhich of these substances will display an incomplete octet in its Lewis structure?
Multiple Choice
CO2
Cl2
ICI
NO
S02

Organic Chemistry
General organic chemistryDuring exercise, muscle cells break down glucose
(C6H12O6) to provide energy. This chemical reaction is
represented by the following chemical equation.
C6H12O6 + 602⇒ 6CO2 + 6H₂O + energy
glucose oxygen carbon dioxide water
How does this equation show the conservation of
mass?
Energy is created by breaking apart molecules.
The mass on each side of the equation is the same.
Mass is conserved when it is converted to heat.
Glucose is broken down into smaller molecules.

Organic Chemistry
General organic chemistryConservation of Mass:Question 1
Which of the following is a correctly written chemical
equation that demonstrates the conservation of
mass?
Mg + HC1 → H+MgCl2
KC103 → KC1+O₂
H₂ + O₂ → H₂O
H₂O+CO₂ → H₂CO3

Organic Chemistry
Chemistry in Daily LifeConservation of Mass:Question 3
Which equation is a balanced chemical
equation?
Select one:
2NaOH+CaBr₂ → Ca(OH)₂ + NaBr
CaCO,→ CaO+2CO,
N+ Cl₂
T NCI
2A1Cl3 + 3Mg 3MgCl₂ + 2A1

Organic Chemistry
Chemistry in Daily LifeWhy is the following statement true or false?
When a match burns, the match goes away, and
thus some matter is destroyed.
False: The mass of ash is the same than the match it
came from.
True: This chemical reaction destroys matter.
True: Matter is consumed by the flame.
False: The atoms are not destroyed, they are only
rearranged.

Organic Chemistry
Chemistry in Daily LifeA. An element with the valence electron configuration 3s²3p¹ would form a monatomic ion with a charge of 1+
In order to form this ion, the element will lose 1 electron(s) from/into the p ✓subshell(s).
B. An element with the valence electron configuration 3s²3p5 would form a monatomic
In order to form this ion, the element will gain 1 electron(s) from/into the p
An error has been detected in your answer. Check for typos,
miscalculations etc. before submitting your answer.
Submit Answer
Retry Entire Group
9 more group attempts remaining
S
P
d
f
s+p
s+d
p+d