Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
General organic chemistryFor each pair of elements label the element from which it is easier to remove an electron.
a. rubidium and strontium
(select)
b. fluorine and neon
(select)
c. nitrogen and arsenic
(select)
d. chlorine and selenium
(select)

Organic Chemistry
IsomerismQuestion 7 (1 point)
For this question, Vibranium (Vb) is a fictional metal in group 2 of the Periodic table.
Write the formula for Vibranium Nitride
Question 8 (1 point)
For this question, Adamantium (A) is a fictional transition metal with multiple
possible charges.
Write the formula for:
Adamantium (II) Hyponitrite

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Some coal is high in sulfur (S) content, and when it burns, it forms sulfuric acid (H₂SO4), a major
component of acid rain, by a series of reactions. Balance the equation for the overall conversion draw
below.
S(s) + O₂(g) + H₂O(1)→ H₂SO4(aq)

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Give the electronic configuration for antimony and then convert it to noble gas notation. (Give the
electronic configuration first and the noble gas notation second with the orbitals in order of increasing
energy.)
antimony:
9

Organic Chemistry
Chemistry in Daily LifeWhich represents the greatest mass of sulfur?
1 gram of sulfur
0.5 mole of sulfur
1 molecule of sulfur
6.02 x 1023 atoms of sulfur
How many moles are in 1.204 x 1024 molecules of CO?
2 moles
6.02 moles
5 grams
1.2 moles

Organic Chemistry
General organic chemistryBe sure to answer all parts.
Identify the element that fits each description.
a. an alkali metal in period 3: [(select)
b. a transition metal in period 5, group 6: (select)
c. a main group element in period 3, group 5A: (select)

Organic Chemistry
General organic chemistryFor the following pairs of elements:
Phosphorus and Barium
i) State if a bond is formed and why (if the answer is no, stop here but EXPLAIN
WHY)
ii) State the type of bond that is formed (if there is a bond)
iii) In your rough notes, if a bond is formed show how this type of bond forms using
Lewis Dot Diagrams

Organic Chemistry
General organic chemistryConsider the following chemicals and answer the questions below
I. F2 II. CH₂Cl2 III. CH3OH
IV. CO2
a. Which chemical has the highest boiling point? (answer using the Roman numeral)
b. Which chemical has the highest vapor pressure? (answer using the Roman numeral)
c. Which chemical is capable of hydrogen bonding with other identical chemicals? (answer using the Roman numeral)
d. How many of the above chemicals can participate in dispersion interaction with other identical chemicals? (answer using a number)
e. How
many of the above chemicals can have dipole-induced dipole interaction with H₂O molecules? (answer using a number)

Organic Chemistry
General organic chemistryYou analyzed 2.81 grams of a mixture of NaCl, SiO2, and CaCO3. You isolate 0.673
grams NaCl, 0.982 grams SiO2, and 0.384 grams CaCO3. What is the percent recovery
for each of the compounds? Round your answer to 2 decimal places.

Organic Chemistry
General organic chemistryTypical atmospheric pressure in Phoenix is 730 mm Hg. Convert this value to (a) atmospheres; (b) psi.

Organic Chemistry
General organic chemistryComplete and balance all of the equations for double displacement reactions below.
Indicate all gases with (g) or T. precipitates with (s) or ↓, and soluble compounds with
(aq). Write "NR" next to the equation if none takes place,
(c) ammonium chloride + sodium nitrate

Organic Chemistry
General organic chemistryBe sure to answer all parts.
The temperature of a 0.50-L gas sample at 25°C and 1.00 atm is changed to each of the following
temperatures. What is the final pressure of the system? The volume and the number of gas particles are
constant.
a. 70.°C
atm
b. 300.°C
atm

Organic Chemistry
Chemistry in Daily LifeHow hot must the air in a balloon be heated if initially it has a volume of 700. L at 20°C and the final
volume must be 1,360. L? The balloon is sealed so that the pressure and number of air particles are
constant.
K

Organic Chemistry
Practical DetectionAn unknown amount of gas occupies 32.5 L at 4.71 atm and 298 K. How many moles does the sample
contain? What is the mass if the gas is helium? What is the mass if the gas is argon?
mol
If the gas is helium:
If the gas is argon:
g

Organic Chemistry
General organic chemistryGiven the following reactants:
KBr + Al2(SO4)3 Ⓡ
a) State the the type of reaction (If there is no reaction, write no reaction, explain
why and stop here).
b) Predict the products and write the complete balanced equation. If you're unable to
balance, still write the unbalanced skeleton equation. Use --> for the arrow.

Organic Chemistry
IsomerismBe sure to answer all parts.
A sample of helium gas has a volume of 2.0 L at a pressure of 4.0 atm. What is the volume of gas at each
of the following pressures? The temperature and the number of gas particles do not change.
a. 5.3 atm
L
b. 11.2 atm

Organic Chemistry
Aldehydes & KetonesConsider the formaldehyde (CH₂O) molecule.
What is the central atom? Enter its chemical symbol.
How many lone pairs are around the central atom? 0
What is the ideal angle between the carbon-hydrogen 0
bonds?
Compared to the ideal angle, you would expect the
actual angle between the carbon-hydrogen bonds to
be...

Organic Chemistry
General organic chemistryWhich section of the Constitution outlines the structure of the legislative
branch of government?
A. The Preamble
B. Article I
C. Article III
D. Article II

Organic Chemistry
General organic chemistryWhich among the following receptors respond to hot and cold temperatures?
Chemoreceptors
Mechanoreceptors
Pain receptors
Thermoreceptors

Organic Chemistry
General organic chemistryThe empirical formula of a compound is NO₂. Its molecular mass is 46 amu.
What is the molecular formula of the compound?
Select the correct answer below:
NO₂
NO
N₂O4
N408

Organic Chemistry
General organic chemistryWhen a sample is placed in the NMR machine,
The majority of magnetic fields of all protons present align with the magnetic field of the
machine, and a small percentage aligns against the field of the machine.
The entirety of magnetic fields of protons present align with the magnetic field of the
machine.
The entirety of magnetic fields of protons aligns against the magnetic field of the machine.
The minority of magnetic fields of all protons present align with the magnetic field of the
machine, and a large percentage aligns against the field of the machine.

Organic Chemistry
General organic chemistryA 20.0 g sample of a hydrocarbon is found to contain 2.86 g hydrogen. What
is the percent by mass of carbon in the hydrocarbon?
Select the correct answer below:
85.7% carbon
14.3% carbon
50.0% carbon
61.8% carbon

Organic Chemistry
General organic chemistryWhat does the law of definite proportions state?
Select the correct answer below:
All samples of a pure compound contain the same elements in the same proportion by mass.
When two elements react to form more than one compound, a fixed mass of one element will react with masses of
the other element in a ratio of small, whole numbers.
For every action there is an equal and opposite reaction.
The total energy of the universe is constant.

Organic Chemistry
HydrocarbonsIf we compare a mole of carbon atoms to a mole of oxygen atoms, which
sample will contain more atoms?
Select the correct answer below:
the carbon sample
the oxygen sample
they will contain the same number of atoms
impossible to tell
|||

Organic Chemistry
General organic chemistryWhat was Jacob Riis's goal in the late 1800s?
to expose corruption in city governments
to start settlement houses for the less fortunate
to preach a gospel of social justice for the less fortunate
to make Americans aware of problems in cities
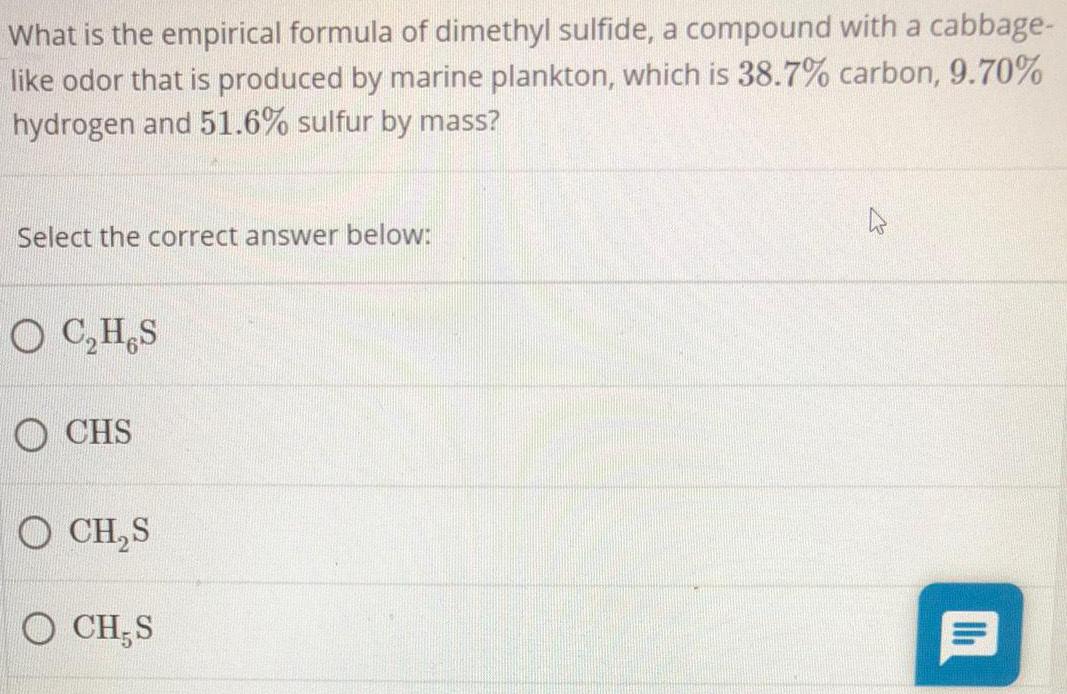
Organic Chemistry
General organic chemistryWhat is the empirical formula of dimethyl sulfide, a compound with a cabbage-
like odor that is produced by marine plankton, which is 38.7% carbon, 9.70%
hydrogen and 51.6% sulfur by mass?
Select the correct answer below:
C₂H S
CHS
CH₂S
CH S

Organic Chemistry
General organic chemistryWhich is a difference between molecular compounds and ionic compounds?
Select the correct answer below:
Molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form
between nonmetals.
Molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds
result from the sharing of electrons between neutral atoms.
Molecular compounds are formed of discrete, neutral atoms, while ionic compounds are formed of large repeating
arrays
of opposite charges.
Molecular compounds have high melting points and high boiling points, while ionic compounds are typicaly found
as gases or low-melting solids and low-boiling liquids.

Organic Chemistry
General organic chemistryWhich is NOT a property common to ionic compounds?
Select the correct answer below:
In solid form ionic compounds are not electrically conductive.
Most ionic compounds are liquids.
Most ionic compounds have very high boiling points.
lonic compounds are electrically conductive in the molten state.

Organic Chemistry
HydrocarbonsA 10.0 g sample of a compound is found to contain 1.00 g carbon, 0.840 g
hydrogen, and 8.16 g chlorine. What is the compound's percent composition?
Select the correct answer below:
19.4% carbon, 31.9% hydrogen, 48.7% chlorine
51.0% carbon, 22.7% hydrogen, 26.3% chlorine
1.00% carbon, 0.840% hydrogen, 8.16% chlorine
10.0% carbon, 8.40% hydrogen, 81.6% chlorine

Organic Chemistry
General organic chemistryThe tires on an off-road bike are inflated to 36 psi. Convert this value to (a) atmospheres; (b) mm Hg.

Organic Chemistry
General organic chemistry2. Draw the reaction-energy diagram for the addition of HBr to 3-methyl-1,2-pentadiene. Make sure to
show both possible pathways the reaction may undergo.
Fill in the structures of reactants, intermediates and products on the diagram.
Brieflty explain the differences in the pathways.

Organic Chemistry
General organic chemistryWhat is NOT true about molecular compounds?
Select the correct answer below:
They are also called covalent compounds.
They are typically formed between nonmetals.
They result when atoms transfer electrons to form ions.
They typically exist as gases, low-boiling liquids, and low-melting
solids.

Organic Chemistry
Carboxylic acids10. Starting from benzene, provide a reasonable synthesis scheme to produce each of the following
compounds.
Make sure to include any necessary reagents and intermediates.
DO NOT DRAW THE MECHANISMS HERE.

Organic Chemistry
General organic chemistryHow many moles of air are present in the lungs if they occupy a volume of 4.7 L at 37°C and
743 mm Hg? How many molecules of air does this correspond to?

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Calculate the volume of each substance at STP.
a. 4.8 mol Ar
L
b. 1.3 g CO₂
L

Organic Chemistry
Amines5. Write the condensed (or line-angle) structural formula for the following compounds:
a. 2,2-dimethyl-1-propanamine
b. 4-methyl-2-pentanamine
c. N-isopropyl-2-butanamine
d. 2-bromoaniline
e. N-cyclohexylethanamide
f. benzamide
g. N-methylhexanamide
h. N-butyl,N-methylpropanamide

Organic Chemistry
Practical DetectionA sample of a compound is 30.9% Na, 21.5% O, and 47.6% Cl by mass.
What is the empirical formula of this compound?
Select the correct answer below:
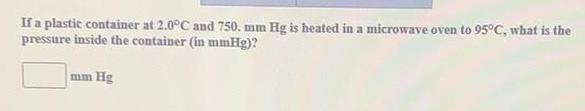
Organic Chemistry
Practical DetectionIf a plastic container at 2.0°C and 750. mm Hg is heated in a microwave oven to 95°C, what is the
pressure inside the container (in mmHg)?
mm Hg

Organic Chemistry
General organic chemistryConvert both values in the blood pressure reading 130/90 to atmospheres.
130 mm Hg =
90 mm Hg =
atm
atm

Organic Chemistry
Practical DetectionThe following excerpt is taken from The Scarlet Letter. Do the words explain the narrator's
reaction to the letter "A."
"It seemed to me then, that I experienced a sensation not altogether physical, yet almost so, as of
burning heat, and as if the letter was not of red cloth, but red-hot iron. I shuddered, and
involuntarily let it fall upon the floor."
Which among the following ideas is explicitly stated in the given lines?
Letter A is used as a means to humiliate sinner...
The narrator is made to feel guilty for her sin.
Letter A is Imprinted on the narrator's body.
Letter A is voluntarily worn by the narrator.

Organic Chemistry
Practical DetectionEnter your answer in the provided box.
A volume (39.0 L) of gas at 52 K is heated to 520 K. What volume does the gas (in Liters) now occupy?
(The pressure and the number of particles do not change.)

Organic Chemistry
General organic chemistry2. Draw the molecular orbital energy diagram for: N₂²+ (strong s-p interaction) Label all energy levels
(atomic and molecular orbitals). Give the electron configuration, bond order, and state the magnetic properties

Organic Chemistry
General organic chemistryWhich of the following statements is/are true?
a) To convert from one resonance structure to another, only electrons can be moved.
b) of the possible bonds between nitrogen atoms, a double bond is stronger than a triple bond.
c) A non-polar bond will form between two identical atoms of equal electronegativity.

Organic Chemistry
General organic chemistryWhat is the molecular formula of a compound that contains 10.0 g carbon,
1.68 g hydrogen, and 13.4 g oxygen? The molar mass of this compound is
180.0 g/mol.

Organic Chemistry
General organic chemistryCarefully look over the collection of everyday products in the room. Record the names of
organic compounds you see listed. Common names are often used instead of IUPAC names
on product labels. Even so, some of the prefixes and suffixes should be recognizable.

Organic Chemistry
General organic chemistrySodium acetate is used in commercial hand-warming "hot packs". Its chemical
formula is NaC₂H₂O₂. What is the formula mass of sodium acetate?
Select the correct answer below:
52.011 amu
91.101 amu
82.034 amu
70.023 amu
F

Organic Chemistry
General organic chemistryIf two molecules have the same structural formula, what is true about them?
Select the correct answer below:
They have the same molecular formula.
They have different molecular formulas.
They have different empirical formulas.
Their atoms are connected differently.

Organic Chemistry
Reactions of benzeneWhen benzene is treated with methyl chloride and aluminum trichloride under conditions that favor trialkylation, one major
product is obtained.

Organic Chemistry
Practical DetectionEnter your answer in the provided box.
The combustion of coal with oxygen forms CO₂ and releases 94 kcal of energy.
C(s) + O₂(g) → CO₂(g)
How much energy is released when 4.3 mol of O₂ reacts?
ΔH = -94 kcal/mol
kcal of energy released
Report answer to appropriate number of significant figures.

Organic Chemistry
General organic chemistryGiven that 4 NH3 + 5 O2 → 4 N+ 6 H₂O, if 3.00 mol NH3 were made to react with
excess of oxygen gas, the amount of H2formed would be:
3.5 moles
5.4 moles
4.5 moles
6.00 moles
2.5 moles