Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.
![Fats, such as butter, and oils, such as corn oil, are formed from compounds called fatty acids, one of
which is linolenic acid (C18H300₂). Linolenic acid undergoes reactions with hydrogen and oxygen to
form the products shown in each equation.
[1]
C18H30 0₂ + H₂ → C18H3602
linjenic acid
[2] C18H300₂ + O₂ CO₂ + H₂O
linolenic acid
a. Balance Equation [1], which shows the reaction with hydrogen.
b. How many grams of product are formed from 87.0 g of linolenic acid in Equation [1]?
C18H360₂](https://media.kunduz.com/media/sug-question/raw/52736603-1658949821.996188.jpeg?w=256)
Organic Chemistry
General organic chemistryFats, such as butter, and oils, such as corn oil, are formed from compounds called fatty acids, one of
which is linolenic acid (C18H300₂). Linolenic acid undergoes reactions with hydrogen and oxygen to
form the products shown in each equation.
[1]
C18H30 0₂ + H₂ → C18H3602
linjenic acid
[2] C18H300₂ + O₂ CO₂ + H₂O
linolenic acid
a. Balance Equation [1], which shows the reaction with hydrogen.
b. How many grams of product are formed from 87.0 g of linolenic acid in Equation [1]?
C18H360₂
Organic Chemistry
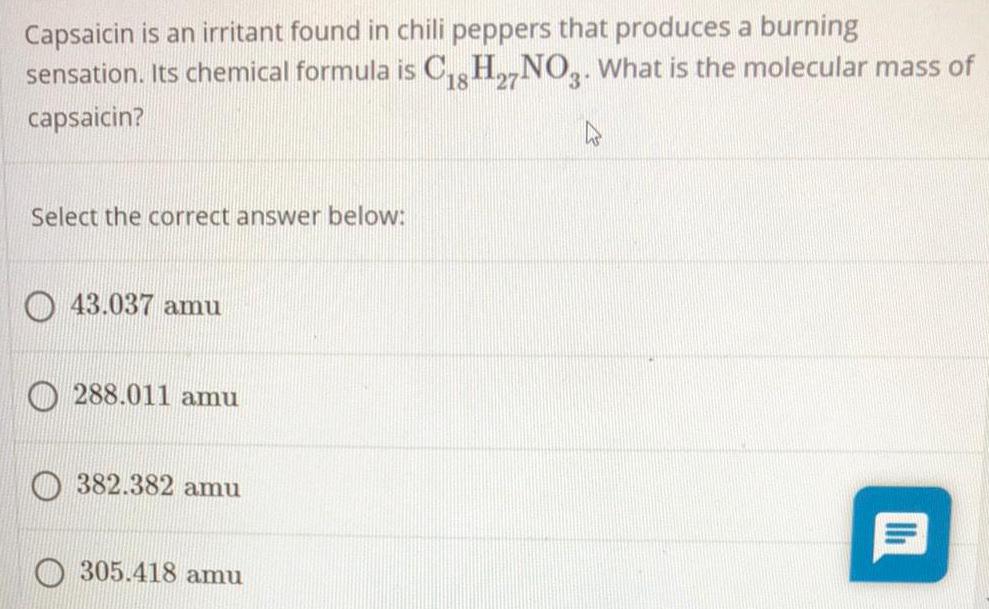
General organic chemistryCapsaicin is an irritant found in chili peppers that produces a burning
sensation. Its chemical formula is C18H27NO3. What is the molecular mass of
capsaicin?
Select the correct answer below:
43.037 amu
288.011 amu
382.382 amu
305.418 amu

Organic Chemistry
General organic chemistryHow many moles are contained in each number of grams of fructose (C6H1206, molar mass
180.2 g/mol), a carbohydrate that is about twice as sweet as table sugar? "Lite" food products use half
as much fructose as table sugar to achieve the same sweet taste, but with fewer calories. Enter your
answer in scientific notation.
a. 12.0 g
b. 0.0630 g

Organic Chemistry
General organic chemistryThe reaction of magnesium metal (Mg) with oxygen (O₂) forms MgO. Write a balanced equation for
this redox reaction. Write two half reactions to show how many electrons are gained or lost by each
species.
Redox Reaction:
Oxidation Reaction:
Reduction Reaction:
-→>

Organic Chemistry
General organic chemistry"So I opened it--you cannot imagine how stealthily, stealthily-until, at length a single dim
ray, like the thread of the spider, shot from out the crevice and fell full upon the vulture
eye."
What things are compared in the line above?
4
Ray of light and the thread of spider
Feeble movement of the narrator and the vulture eye
Movement of the old man and the vulture eye
Movement of the spider and the vulture eye

Organic Chemistry
General organic chemistryHydrocarbons are compounds that contain only C and H atoms. When a hydrocarbon reacts with O₂,
CO₂ and H₂O are formed. Write a balanced equation for the combustion of the following hydrocarbon,
a high-octane component of gasoline. Do not include states of maver in your answer.
C6H, (benzene)

Organic Chemistry
Practical DetectionUpon reading the story, The Scarlet Letter, which among the following was strictly followed in
Puritan society that Hester violated?
1. Illicit affair
II. Refusing to name her child's father
III. Not adhering to all Christian practices
I and II
II and III
I and III
I, II, and III

Organic Chemistry
General organic chemistryA compound has a molecular weight of 318.31 g/mol and an empirical formula of C10 H7O2. What is the molecular formula?
Select the correct answer below:
C20 H1404
C₂H1003
C10 H702
none of the above

Organic Chemistry
General organic chemistryWhich musical form originated in Italy around 1520 A.D.?
The madrigal
The galliard
The passamezzo
Word painting

Organic Chemistry
Practical DetectionDefine Rococo.
A very ornamental style of art that originated in Spain in the early 18th century
A very ornamental style of art that originated in France in the early 16th century
A very ornamental style of art that originated in Austria in the early 14th century
A very ornamental style of art that originated in France in the early 18th century

Organic Chemistry
General organic chemistryDiatomic N2 can react with diatomic H2 to form ammonia (NH3). The balanced chemical equation is:
N2 + 3 H2-2 NH3
If 6 moles of H2 totally reacted with more than enough N2, how many moles of ammonia would be
expected to form?
5 moles
3 moles
6 moles
4 moles
2 moles

Organic Chemistry
Practical DetectionWhich terms are examples of musical texture?
monophonic, polyphonic, homophonic
alto, bass, baritone
soprano, mezzo-soprano, contralto
repetition, contrast, variation

Organic Chemistry
General organic chemistrySuppose two chemical reactions are linked together in a way that the O₂ produced in the
first reaction goes on to react completely with Mg to form MgO in the second reaction.
Reaction one: 2 KCIO3 → 3 O2 + 2 KCI
Reaction two: 2 Mg + O₂ → 2 MgO
If you start with 4 moles of KCIO3, how many moles of MgO could eventually form?
12.0 moles
3.0 moles
6.0 moles
2.0 moles
4.0 moles

Organic Chemistry
Practical DetectionThe cytoplasmic extensions that conduct a received stimulus towards the cell body are called
Schwann cells
dendrons
axons
myelin sheaths

Organic Chemistry
General organic chemistryWhat is the formula mass of a compound with 2 carbon atoms and 3 oxygen
atoms, in atomic mass units?

Organic Chemistry
General organic chemistryWhat is the limiting reactant for the following reaction given we have 3.4 moles of
Ca(NO3)2 and 2.4 moles of Li3PO4?
Reaction: 3Ca(NO3)2 + 2Li3PO4 → 6LINO3 + Ca3(PO4)2
LINO3
CA3(PO4)2
Ca(NO3)2
Li3PO4

Organic Chemistry
General organic chemistryIn comparing a balloon containing 25 grams of helium to a balloon containing 25 grams of
neon, which one of the following statements is TRUE?
Each balloon has an equal number of atoms.
The helium balloon has more atoms.
This scenario cannot happen because gases have no mass.
The neon balloon has more atoms.

Organic Chemistry
General organic chemistryA 17.10 g compound is made up of 5.900 g N and 11.20 g C. What is the
percent composition of carbon in this compound?
• Your answer should have four significant figures.

Organic Chemistry
General organic chemistryWhich of the following equations is NOT balanced properly?
4NH3 + 1402 → 4NO2 + 6H₂O
Cr₂(SO4)3 +6KOH → 2Cr(OH)3 + 3 K2SO4
2NaHCO3 → Na2CO3 + CO2 + H₂O
2Cr + 6HCI →→2CrCl3 + 3H2

Organic Chemistry
Practical DetectionA compound has a molecular weight of 477.18 g/mol and an empirical formula of C10 H7O2. What is the molecular
formula?
Select the correct answer below:
C30 H2106
C4H1303
C14H1203
none of the above

Organic Chemistry
Chemistry in Daily LifePoints 1
Identify the property of ionic compounds.
Insoluble in water.
Brittle
Low melting point.
Low boiling point.

Organic Chemistry
Practical DetectionEnter your answer in the provided box.
Using the balanced equation for the combustion of ethanol, answer the following question.
C₂H6O(l)+3 O₂(g) → 2 CO₂(g) + 3 H₂O(g)
ethanol
How many grams of CO₂ are formed from 0.70 mol of ethanol?

Organic Chemistry
General organic chemistryWhat is the formula mass of a compound with 2 aluminum atoms, 3 sulfur
atoms, and 3 oxygen atoms, in atomic mass units?

Organic Chemistry
Practical DetectionBe sure to answer all parts.
MTBE (C5H12O) is a high-octane gasoline additive with a sweet, nauseating odor. Because small
amounts of MTBE have contaminated the drinking water in some towns, it is now banned as a fuel
additive in many areas. MTBE reacts with O₂ to form CO₂ and H₂O. Write a balanced equation for the
combustion of MTBE. Do not included states of matter in your answer.

Organic Chemistry
Practical DetectionBe sure to answer the parts.
How many molecules are contained in each of the following number of moles?
Report your answer using correct significant figures.
a. 0.89 mol of sugar molecules
b. 57.5 mol of acetaminophen molecules

Organic Chemistry
General organic chemistryWhat types of elements are predominantly present in the periodic table?
Metalloids
Metals
Nonmetals
Nonmetals and metalloids

Organic Chemistry
General organic chemistryHow many moles of lithium nitrate are theoretically produced if we start with 3.4 moles of
Ca(NO3)2 and 2.4 moles of Li3PO4?
Reaction: 3Ca(NO3)2 + 2Li3PO4 →6LINO3 + Ca3(PO4)2
6.8
8.1
7.2
2.8
1.2

Organic Chemistry
HydrocarbonsFree radicals are defined as (A) with (B) one unpaired electron.
A: molecules B: at least
A: atoms B: at least
A: molecules or atoms B: at least
A: ions, molecules, or atoms B: at least
A: ions B: at most
A: molecules or ions B: at least
A: molecules, atoms, or ions B: at most

Organic Chemistry
Practical DetectionThese words are spoken by Hester to Dimmesdale when the townspeople wanted to separate
Hester and her child.
"Speak thou for me!' cried she. Thou wast my pastor, and hadst charge of my soul, and knowest me
better than these men can. I will not lose the child! Speak to me! Thou knowest, for thou hast
sympathies which these men lack! - thou knowest what is in my heart, and what are a mother's
rights, and how much the stronger they are when that mother has but her child and the scarlet
letter! Look thou to it! I will not lose the child! Look to it!"
Which among the following lines/phrase thát Hester speaks to convey a subtle message to
Dimmesdale?
"Speak thou for meľ' cried she."
"Thou knowest, - for thou hast sympathies which these men lack!"
'Thou knowest what is in my heart"
"How much the stronger they are, when that mother has but her child and the scarlet letter!"

Organic Chemistry
General organic chemistryNitrogen dioxide, NO₂, also an undesired product formed during combustion, is converted to N₂ and O₂
in a catalytic converter. Write a balanced equation for this reaction.

Organic Chemistry
General organic chemistryConsider the balanced equation of KI reacting with Pb(NO3)2 to form a precipitate.
2 KI (aq) + Pb(NO3)2 (aq) → PbI₂ (s) + 2 KNO3(aq)
What mass of PbI₂ can be formed by adding 0.537 L of a 0.329 M solution of KI to a solution of excess Pb(NO3)2?

Organic Chemistry
HydrocarbonsFree radical are
in general, compared to non-radicals.
low in energy, unreactive, and stable.
low in energy, reactive, and unstable.
high in energy, reactive, and unstable.
high in energy, reactive, and stable.
high in energy, unreactive, and unstable.

Organic Chemistry
Practical DetectionPoints 2
The dominant intellectual movement of the Renaissance was called
Romanticism
Feudalism
Humanism
Classicism

Organic Chemistry
General organic chemistryGiven the balanced equation CO₂ + Si → SiO2 + C, if you were to react 1 mole of CO2 with
1 mole of Si, which statement is TRUE?
The SiO₂ is the limiting reactant.
The CO₂ is the limiting reactant.
The Si is the limiting reactant.
You have equal stoichiometric amounts of reactants.

Organic Chemistry
General organic chemistryUsing molarity to find solute moles and solution volume
A chemist adds 140.0 mL of a 0.49 mol/L aluminum chloride (AICI) solution to a reaction flask. Calculate the millimoles of aluminum chloride the chemist
has added to the flask. Be sure your answer has the correct number of significant digits.

Organic Chemistry
General organic chemistryWhich of the following Renaissance compositions provides an excellent example of word painting?
The Kyrie from Glovannie Pierluigi da Palestrina's Pope Marcellus Mass
Successores by Hildegarde of Bingen
The Terpsichore collection by Michael Praetorious
The madrigal "As Venus Was Descending" by Thomas Weelkes
![Balance the equation for the formation of magnesium hydroxide [Mg(OH)₂], one of the active
ingredients in milk of magnesia. Do not include states of matter in your answer.
MgCl₂ + NaOH → Mg(OH)₂ + NaCl](https://media.kunduz.com/media/sug-question/raw/52736611-1658948106.3335598.jpeg?w=256)
Organic Chemistry
General organic chemistryBalance the equation for the formation of magnesium hydroxide [Mg(OH)₂], one of the active
ingredients in milk of magnesia. Do not include states of matter in your answer.
MgCl₂ + NaOH → Mg(OH)₂ + NaCl

Organic Chemistry
General organic chemistryQuestion 10
Contrast these characteristics to determine which styles it is referring to; music, acting, poetry,
dance, scenery, costumes in a theatrical performance versus large-scale composition for chorus,
vocal soloists, orchestra, set to a narrative text but have no acting, scenery, or costumes.
Points 2
Opera versus Cantata
Opera versus Oratorio
Oratorio versus Cantata
Oratorio versus Suite
Complete Later
Complete

Organic Chemistry
General organic chemistryAs a result of free-radical mono-halogenation of alkanes, one of the (A) of alkane is (B) by a (C).
A: hydrogens B: eliminated C: halogen atom.
A: hydrogens B: substituted C: halogen atom.
A: halogens B: substituted C: hydrogen atom.
A: halogens B: eliminated C: hydrogen atom.
Organic Chemistry
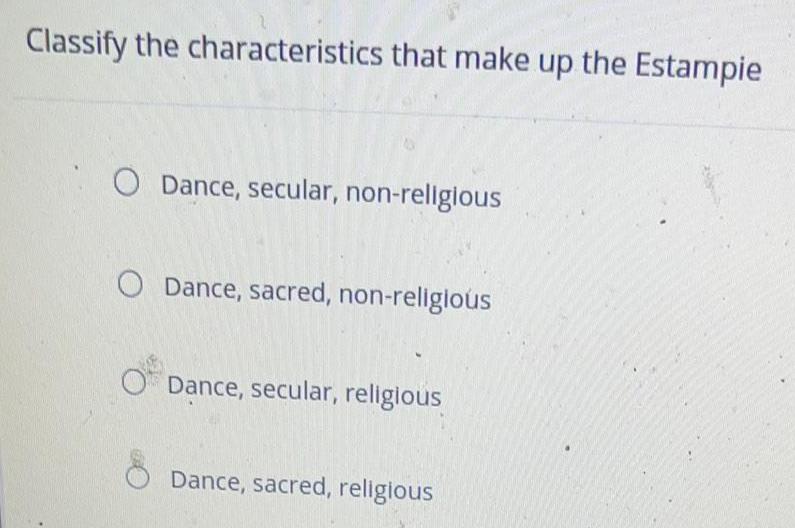
Practical DetectionClassify the characteristics that make up the Estampie
Dance, secular, non-religious
Dance, sacred, non-religious
Dance, secular, religious
Dance, sacred, religious
Organic Chemistry
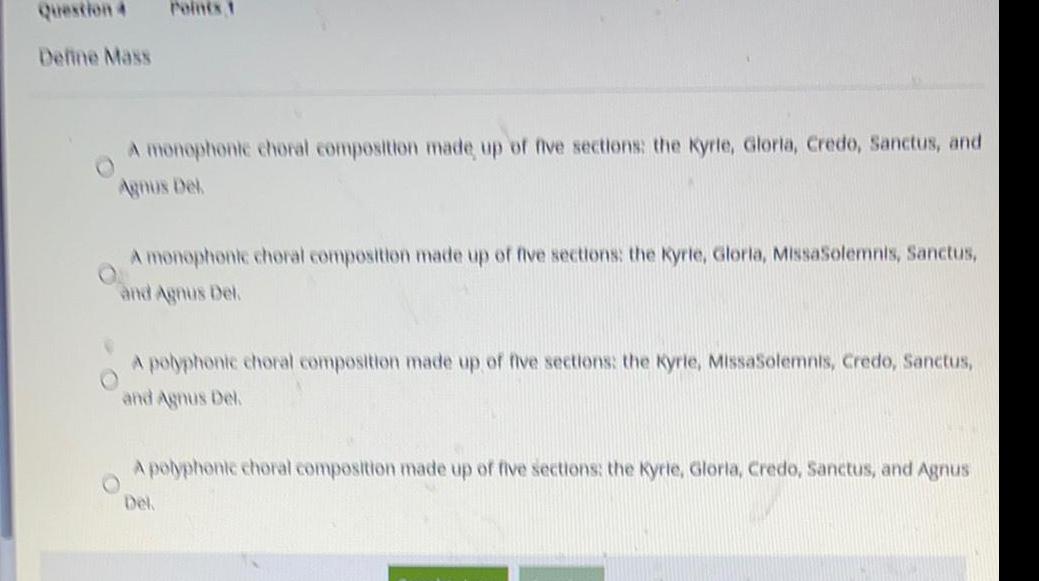
General organic chemistryQuestion 4
Define Mass
Points 1
A monophonic choral composition made up of five sections: the Kyrle, Gloria, Credo, Sanctus, and
Agnus Dek
A monophonic choral composition made up of five sections: the Kyrie, Gloria, MissaSolemnis, Sanctus,
and Agnus Del.
A polyphonic choral composition made up of five sections: the Kyrie, MissaSolemnis, Credo, Sanctus,
and Agnus Del.
A polyphonic choral composition made up of five sections: the Kyrie, Gloria, Credo, Sanctus, and Agnus

Organic Chemistry
Chemistry in Daily LifeBe sure to answer all parts.
Calculate the number of grams contained in each of the following number of moles. Report your answer
with the appropriate number of significant figures.
a. 0.150 mol of NaCl (sodium chloride)
b. 6.20 mol of C₂H4 (ethylene)
g

Organic Chemistry
General organic chemistryEnter your answer in the provided box.
Use the balanced equation for the reaction of N, and O₂ to form NO to answer the question.
N₂(g) + O₂(g) →→→ 2 NO(g)
How many moles of O₂ are needed to completely react with 6.1 moles of N₂?
mol O₂

Organic Chemistry
General organic chemistryZinc-silver oxide batteries are used in cameras and hearing aids. Identify the species that is oxidized
and the species that is reduced in the following redox reaction. Identify the oxidizing agent and the
reducing agent. Oxide (02) is unchanged in this reaction.
Zn + Ag₂O → ZnO + 2 Ag
Zn: (select)
Ag: (select)
(select)
(select)

Organic Chemistry
General organic chemistryWhich statement below concerning the photoelectric effect is true?
View Available Hint(s)
Increasing the intensity (amplitude) of the light shining on a metal surface is sufficient to cause the emission of electrons from a metal surface.
A threshold wavelength exists for each metal, and only light of a longer wavelength than this threshold causes electrons to be emitted from the metal
surface.
Joy
A threshold frequency exists for each metal, and only light of a frequency higher than this threshold causes electrons to be emitted from the metal
surface.
Light with a frequency below the threshold frequency can still cause electrons to be emitted from the metal surface, but a lag time occurs while
sufficient energy to dislodge the electrons builds up.

Organic Chemistry
BiomoleculesQ2 What is the type of glycosidic bond in maltose?
Q3 Why does maltose have both a and ß forms (anomers)? Explain.
C. Polysaccharides
1.Amylose
What is the difference in the structure of amylopectin and amylose?

Organic Chemistry
Practical DetectionAir is a mixture of nitrogen, oxygen, and argon
(and a few other trace gases that we will ignore
here). Assume you have a container of air,
where nitrogen has a partial pressure of 761
torr, oxygen has a partial pressure of 226 torr,
and argon has a partial pressure of 37 torr.
What is the total pressure in the container?
Your Answer:
![A sample of calcium carbonate [CaCO3 (s)] absorbs 40.3 J of heat, upon which the temperature of
the sample increases from 20.8 °C to 27.3 °C. If the specific heat of calcium carbonate is 0.82 J/g-
K, what is the mass (in grams) of the sample?
-7.6
7.6
5.1
5.3
0.13](https://media.kunduz.com/media/sug-question/raw/52516953-1658947478.274892.jpeg?w=256)
Organic Chemistry
Practical DetectionA sample of calcium carbonate [CaCO3 (s)] absorbs 40.3 J of heat, upon which the temperature of
the sample increases from 20.8 °C to 27.3 °C. If the specific heat of calcium carbonate is 0.82 J/g-
K, what is the mass (in grams) of the sample?
-7.6
7.6
5.1
5.3
0.13

Organic Chemistry
Practical DetectionCalculate the work done in kJ during a reaction in which the internal volume contracts from 48 L to
19 L against an outside pressure of 2.5 atm. (1 L-atm = 101.3 J)
-7300
7.3
7300
0, no work is done.
-7.3

Organic Chemistry
General organic chemistryWhich literary device(s) would be
best to include as part of a
literary analysis of this excerpt
from "The Lady or the Tiger"?
A. The device of understatement, which several
times in the text and supports the theme
B. The device of imagery, which several times
in the text and supports the theme
C. The device of onomateopia, which occurs
once in the text and does not support the theme