Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
Chemistry in Daily LifeQuestion 2 of 25
How can an unknown ΔHreaction be determined using Hess's law?
A. The free energy of the reaction is used to determine the AH for
the reaction.
B. The unknown ΔHreaction is determined after the reaction is run in a
calorimeter.
C. The reaction is repeated at different temperatures to determine
the ΔHreaction-
D. Enthalpies from reaction steps are added to determine an
unknown AHreaction

Organic Chemistry
General organic chemistryQuestion 9 of 10
If an engineer's greatest concern is easy maintenance of a rocket throughout the years, the rocket should be designed to use which type of fuel?
A. Liquid chemical fuel
B. Gaseous fuel
C. Nuclear fuel
D. Solid chemical fuel

Organic Chemistry
Practical DetectionThe density of iron is 7.874.Convert to kilogram per cubic meter.
Do not include units in your answer.
Provide your answer below:

Organic Chemistry
General organic chemistryA chemist prepares a solution of zinc oxalate (ZnC₂O4) by measuring out 1.01 mg of zinc oxalate into a 100. mL volumetric flask and filling the flask to the
mark with water.
Calculate the concentration in mol/L of the chemist's zinc oxalate solution. Be sure your answer has the correct number of significant digits.
_ mol/L
_x10

Organic Chemistry
Chemistry in Daily LifeThis question is based on the following paragraph.
The back yard was covered in late afternoon sunlight. The leaves danced in the trees, delighted that spring had finally arrived. The tulips were bright slashes of color like a child's crayon drawing. In the garden, the first green shoots were pushing out of the soil to greet the warmth of the sun. Sitting on the porch swing, I drank in the beauty of nature, a delicious treat. Which phrase from the paragraph is an example of a metaphor?
A. Greet the warmth of the sun
B. In late afternoon sunlight
C. Delighted that spring had finally ved
D. The beauty of nature, a delicious treat

Organic Chemistry
General organic chemistryCalculate the number of boron atoms in a 70.0 g sample of tetraborane (B4H10).
Be sure your answer has a unit symbol if necessary, and round it to 3 significant digits

Organic Chemistry
General organic chemistryWhat is ironic about this following passage?
"So you're not killed, as you ought to be, but you're caught, anyway," he cried; "caught fast. Ho, what a jest, Ulrich von Gradwitz snared in his stolen forest. There's real justice for you!"
A. Ulrich is caught, helpless in his own forest,
as pointed out by Georg.
B. Georg says, "So you're not killed."
C. Justice is real.

Organic Chemistry
General organic chemistryA catalyst works by.
Select one:
a. changing the particle size of the reactants
b. lowering the activation energy barrier
c. shifting the equilibrium position toward the products
d. changing the temperature of the reactants

Organic Chemistry
Practical DetectionPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains
state symbols after every reactant and product.
HCIO3(aq) + H2o ⇒ _

Organic Chemistry
Practical DetectionIdentify areason that chemical reactions release energy during the reaction process.
Select one:
a. forming bonds
b. breaking bonds
c. storing energy
d. overcoming activation energy

Organic Chemistry
General organic chemistrySituational irony occurs when what is expected to happen is different from what actually happens. From the highlighted passage in Sec. 33, we expect that...
A. They are seeing things because they have been trapped for so long.
B. Some foresters are coming to their rescue.
C. The men's cries won't be heard and they will be passed by.

Organic Chemistry
General organic chemistryWhat drives spontaneous reactions?
Select one:
a. increasing enthalpy and increasing entropy
b, increasing enthalpy and decreasing entropy
c. decreasing enthalpy and decreasing entropy
d. decreasing enthalpy and increasing entropy

Organic Chemistry
Qualitative analysisMethane and chlorine react to form chloromethane, CH3Cl, and hydrogen chloride. When 29.8 g of methane and 40.3 g of chlorine gas undergo a reaction that has a 64.7% yield, how many grams of chloromethane form?
A)60.7 g
B)44.4 g
C)28.7 g
D)18.6 g
E)93.9 g

Organic Chemistry
Hydrocarbons1.49 moles of hydrogen gas are reacted with excess carbon monoxide according to the reaction below. If 15.5 grams of methanol (CH₂OH) are produced, what was the percent yield?
2 H₂(g) + CO(g) → CH₃OH(l)
(A) 59.1 %
(B) 64.9 %
(C) 68.5 %
(D) 72.3 %
(E) 79.19%
(F) 45.9 %

Organic Chemistry
Halogen DerivativesWhich experimental conditions would maximize the yield of choloroethane and minimize the production of polychlorinated products during the free radical chlorination of ethane?
A. equimolar amounts of chlorine & ethane
B. use an excess of chlorine
C. excess of ethane
D. excess of chlorine & long reaction time
E. equimolar amounts of chlorine & ethane and high reaction temperature

Organic Chemistry
Chemistry in Daily LifeEnthalpy change is the
Select one:
a. pressure change of a system at constant temperature.
b. entropy change of a system at constant pressure.
c. temperature change of a system at constant pressure.
d. amount of energy absorbed or lost by a system as heat during a process at constant pressure.

Organic Chemistry
General organic chemistryQuestion 1 of 10
When is a secondary source more helpful than a primary source?
A. When you are an expert in the field being studied in the experiment
B. When you want all the details of the experiment
C. When you want a quick summary of the experiment
D. When you want to perform the experiment yourself

Organic Chemistry
General organic chemistryWhich situation is
the best example of
irony?
A. The map showed me
where to go to get to my
destination.
B. I got extremely thirsty
while swimming in the pool.
C. Her family photo album
has pictures of her family
and also all of her friends.

Organic Chemistry
General organic chemistryWhich is the best summary
of paragraph 8 of "The Lady,
or the Tiger?"
A. The crowd likes to see someone be
punished. They come often to the arena.
B. The crowd was usually at a hilarious
wedding.
C. The surprise verdict makes the arena
popular. Because the choice is the
accused's, it seems just.

Organic Chemistry
General organic chemistryWhich of the following is the
of
best summarization
paragraphs 23 and 24?
A. The princess is weighing her options.
B. The princess is wondering where her
lover is.
C. The princess is waiting for something
to happen.

Organic Chemistry
General organic chemistryQuestion 5 of 10
How is heat transferred from one object to another?
A. Heat moves from warmer objects to cooler objects.
B. Heat moves between objects of the same temperature.
C. Heat moves back and forth between two objects.
D. Heat moves from cooler objects to warmer objects.

Organic Chemistry
Chemistry in Daily LifeIdentify each category of substance as soluble or insoluble in water.
Salts of Group 1 elements Choose...
Most nitrate salts Choose...
Most carbonate and phosphate salts Choose...
Most halide (Br, Cl, and I) salts Choose...
Most silver salts Choose...

Organic Chemistry
Chemistry in Daily LifeAn aqueous solution at 25 °C has a pOH of 12.62. Calculate the pH. Be sure your answer has the correct number of significant digits.

Organic Chemistry
General organic chemistryA chemist prepares a solution of copper(II) sulfate (CuSO4) by measuring out 51. g of copper(II) sulfate into a 400. mL volumetric flask and filling the flask
to the mark with water.
Calculate the concentration in mol/L of the chemist's copper(II) sulfate solution. Be sure your answer has the correct number of significant digits.

Organic Chemistry
Chemistry in Daily LifeIn the story of Icarus, the
Exposition (establishing the
setting and the conflict of the
story) is
A. the explanation that Icarus and his
father are prisoners and want to escape.
B. the detail of Icarus falling into the sea.
C. the details of how Icarus' father made
the special wings.

Organic Chemistry
General organic chemistryThe lab you have been assigned in chemistry class asks you to mix two chemicals together to create a chemical
reaction. You do not have much time to complete the lab. Which of these would help speed up the reaction rate?
Select one:
a. put the chemicals on a ice bath
b. carefully heat the chemicals on a hot plate
c. do not add the supplied catalyst to the reaction
d. add more solvent to the chemicals in order to lower their concentration

Organic Chemistry
Chemistry in Daily LifeWhat is the mass of potassium sulfate in 500 mL of a 1.25 M potassium sulfate solution?
We know the volume (500 mL or 0.5 L) and we know the moles per liter, 1.25 M, but we need mass.
Solution:
• First, determine how many moles we have in solution:
125 x 0.500 L = 0.625 mol
• Then, convert that amount to grams. To do this you must get the molar mass of K,SO, from the periodic table 174,3 g/mol. Use the number of moles you calculated above to find grams of potassium sulfate
W
0.625 mol x
1743 g K, SO,
mal K, 50,
= 109 g K₂ SO4
Therefore, the mass of the K₂SO, that dissolves in 500 mL of H₂O to make this solution is 109 g.
Solve this problem on paper, then submit your answer to see the solution: How many grams of Ca (OH), are needed to make 100.0 mL of 0.250 M solution?
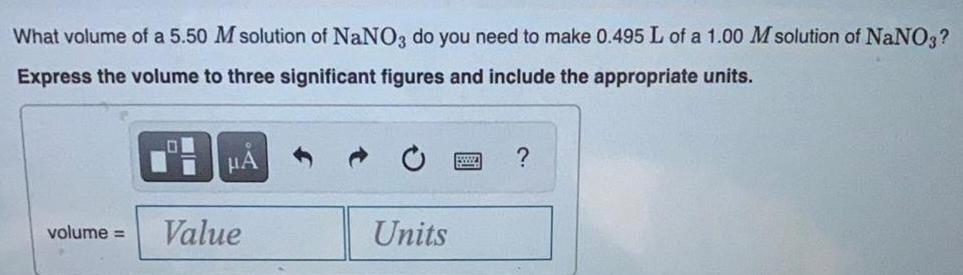
Organic Chemistry
General organic chemistryWhat volume of a 5.50 M solution of NaNO3 do you need to make 0.495 L of a 1.00 M solution of NaNO3?
Express the volume to three significant figures and include the appropriate units.

Organic Chemistry
Chemistry in Daily LifeAll BUT one action would increase the rate at which solid metal will react with an acid solution. That is
Select one:
a. decrease the molarity of the acid.
b. break the metal into smaller pieces.
c. increase the temperature of the solution.
d. increase the concentration of the acid solution.

Organic Chemistry
General organic chemistryCalculate the mass of camphor (C₁0H₁60) that contains a trillion (1.0 × 10¹2) carbon atoms.
Be sure your answer has a unit symbol if necessary, and round it to 2 significant digits.

Organic Chemistry
General organic chemistryCalculate the mass of forsterite (Mg₂SiO4) that contains a billion (1.0 × 109) magnesium atoms.
Be sure your answer has a unit symbol if necessary, and round it to 2 significant digits.

Organic Chemistry
General organic chemistryWhich of the following chemical equations is balanced correctly?
A. Zn(s) + 2AgNO3(aq) → 2Ag(s) + Zn(NO3)2(aq)
B. CH₂OH(1) + 20₂(g) → CO₂(g) + 2H₂O(1)
C. H₂SO4(aq) + 2NaOH(aq) → Na₂SO4(s) + H₂O(l)
D. 2Al(s) + 302(g) → Al₂O3(s)

Organic Chemistry
Chemistry in Daily LifeA sample of a substance burns more rapidly in pure oxygen than in air. Which factor is most responsible for this
high rate of reaction?
Select one:
a. concentration of the substance
b. the properties of the reactants
c. temperature
d. surface area exposed to air
Clear my choice

Organic Chemistry
General organic chemistryWhich sentence uses the
underlined vocabulary word
incorrectly?
A. I filled out the acquiesce for the insurance
company.
B. Tom's pain and his languor let us know he
hadn't completely recovered from his surgery
yet.
C. The two angry men could not shake hands
and come to a reconciliation in a timely manner.

Organic Chemistry
Chemistry in Daily LifeIn a chemical reaction, when particle size is reduced, the rate of reaction increases because of increased surface
area of the reactants. Why would increased surface area increase reaction rate?
Select one:
a. It decreases the activation energy.
b. It increases the number of collisions among particles.
c. It decreases the temperature.
d. It increases the concentration.

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Running at a rate of 7 mi/h uses 812 Calories in an hour. Convert this value to (a) J/h and (b) kJ/h.
Report answers to appropriate number of significant figures.

Organic Chemistry
General organic chemistryBased on the second
definition given, which
sentence most correctly uses
the word academic as
"assumed, a known
procedure"?
A. My parents reward me for my
academic progress -- my grades in
school.
B. An athlete should consider a college's
academic program as well as its sports
program.
C. Getting candy by throwing a fit has
become academic for the child whose
mother gives in.

Organic Chemistry
General organic chemistryIs pentene (Molecular formula: C5H10) saturated, mono-
unsaturated, or poly-unsaturated.
Hint: Draw the Lewis structure
Mono-unsaturated
Poly-unsaturated
Saturated
Depending on how it is drawn, it could be any of the
choices.

Organic Chemistry
General organic chemistryHow does describing the
princess's conflicting feelings
build the tension and the
action?
A. It doesn't - the author should have
described her clothes and her hair color to
build the action.
B. It makes the conflict seem real and
leaves the reader anxious to know what
happens at the climax.
C. The author should have described
what the king felt - he wasn't worried
about what would happen.

Organic Chemistry
Practical DetectionWhat is the net ionic equation of the reaction of ZnCl2 with NaOH?
Express you answer as a chemical equation including phases.
Organic Chemistry
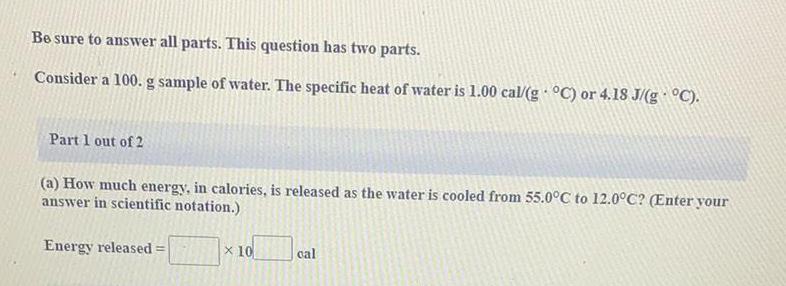
Practical DetectionBe sure to answer all parts. This question has two parts.
Consider a 100. g sample of water. The specific heat of water is 1.00 cal/g °C) or 4.18 J/(g · °C).
Part 1 out of 2
(a) How much energy, in calories, is released as the water is cooled from 55.0°C to 12.0°C? (Enter your
answer in scientific notation.)
Energy released = _ x 10 _ cal

Organic Chemistry
General organic chemistryThe thermochemical equation for the reaction of carbon dioxide with sulfur
dioxide is shown below. How can the reaction be described?
CO₂(g) +2SO₂(g) +1104 kJ → CS₂(g) +30₂(g)
A., It has a low activation energy.
B. It is exothermic.
C. It has a high activation energy.
D. It is endothermic.

Organic Chemistry
General organic chemistryHow much energy in kilocalories is needed to vaporize 185 g of water? The heat of vaporization of
water is 540 cal/g.
kcal

Organic Chemistry
General organic chemistrySelect the single best answer.
Which of the following samples has the higher final temperature?
12.0 g of aluminum at 18°C that absorbs 95.0 cal of heat
16.0 g of iron at 22°C that absorbs 55.0 J of heat

Organic Chemistry
General organic chemistryWhat is ironic about the following
passage?
41- "No," said Ulrich with a laugh,
the idiotic chattering laugh of a
man unstrung with hideous fear.
42- "Who are they?" asked Georg
quickly, straining his eyes to see
what the other would gladly not
have seen.
43- "Wolves."
A. The men were finally ready to end their feud
when their feud resulted in the end of them.
B. Georg was too blind to see their fate.
C. The men had traveled in packs just like the
wolves

Organic Chemistry
General organic chemistryIf 75 kJ of heat is transferred to 1200 g liquid water at 36°C, what would the
new temperature of the water be? (The specific heat capacity of liquid water
is 4.186 J/g °C.)
A. 15°C
B. 51°C
C. 100°C
D. 36°C

Organic Chemistry
General organic chemistryAweather balloon has a volume of 52.5 liters at a temperature of 295 K. The balloon is released and rises to an altitude where the
temperature is 252 K.
A. How does this temperature change affect the gas particle motion? Explain.
B. The original pressure at 295 K was 100.8 kpa and the pressure at the higher altitude at 252 K is 45.6 kpa.
Calculate the volume of the balloon at the higher altitude.

Organic Chemistry
Chemistry in Daily LifeSome dry chemicals can be used to put out forest fires. One of these chemicals is NaHCO3.
When NaHCO3(s) is heated, one of the products is CO₂(g), as shown in the unbalanced equation below.
NaHCO3(s) + heat → Na₂CO3(s) + H₂O(g) + CO₂(g)
A. Balance the above equation using smallest whole number coefficients.
B. Identify the type of chemical reaction represented by the above equation.
C. Draw a potential energy diagram to show that the above reaction is endothermic.

Organic Chemistry
General organic chemistryConsider the following elements: Sodium, Carbon, Calcium, Fluorine, Oxygen.
A. Name two elements that would form an ionic compound.
B. Write the correct formula of an ionic compound that could be formed by the elements you gave in part (A).
C. Give the correct name of the ionic compound you wrote in (B).
D. Propose an experimental test you can perform to prove that the compound you wrote in (B) is ionic.
E. Name an element from the above list that conducts electricity in solid state. Justify.

Organic Chemistry
Chemistry in Daily LifeWhat is entropy a measure of?
A. The maximum potential energy of a system
B. The change in energy of a reaction
C. The disorder of a system
D. The energy available to do work