Chemistry in Daily Life Questions and Answers

Organic Chemistry
Chemistry in Daily LifeCalculate the pressure, in atmospheres, of 8.67 mol CO(g) in a 2.0 L tank at 48 degrees C.

Organic Chemistry
Chemistry in Daily LifeWhy is John Maynard Keynes often considered the savior of liberalism between the two world wars?
a) He suggested a way for government to intervene in private economic matters, because he
recognized that businesses cannot save the economy.
b) He told people that investing in a central bank was a bad idea.
c) He reminded people that inequality can only be cured with individual effort.
d) He urged a greater role for business in a country's economic life.

Organic Chemistry
Chemistry in Daily LifeEach step in the following process has a yield of 70.0%.
CH4 +4Cl₂→ CCI4 + 4HCI
CCI + 2 HF→ CCI₂F₂ + 2HCl
The CCI, formed in the first step is used as a reactant in the second step.
If 8.00 mol CH₂ reacts, what is the total amount of HCI produced? Assume that Cl, and HF are present in excess.
moles HCl: mol

Organic Chemistry
Chemistry in Daily LifeA piece of Zn was added to CuSO4solution. Initially CuSO4 (aq) is a blue/green color and after 30 minutes it turned light blue. Initially the Zn (s) was dull gray and after 30 minutes it was black.
Based on the above observations, answer the following questions:
a. What evidence is there of a chemical reaction?
b. Write a balanced equation for this reaction.
c. What type of chemical reaction is this?

Organic Chemistry
Chemistry in Daily LifeInitially magnesium is a shiny light gray ribbon of material. When placed in flame heat and light energy are produced and a white ash is produced.
Based on the above observations, answer the following questions:
a. What evidence is there of a chemical reaction?
b. Write a balanced equation for this reaction.
c. What type of chemical reaction is this?
Organic Chemistry
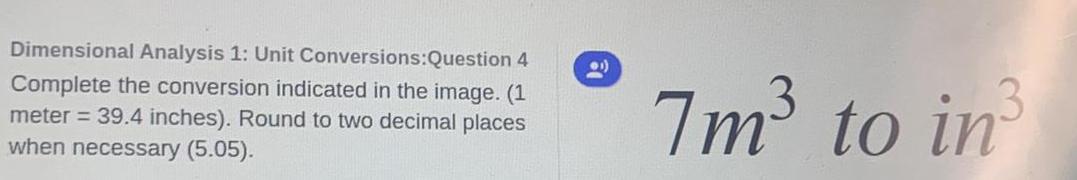
Chemistry in Daily LifeDimensional Analysis 1: Unit Conversions:Question 4
Complete the conversion indicated in the image. (1
meter = 39.4 inches). Round to two decimal places
when necessary (5.05).
1)
7m³ to in³

Organic Chemistry
Chemistry in Daily LifeIn which of these substances are the atoms held together by polar covalent bonding?
Multiple Choice
SrCl2
CsCI
CIF
TIF2
S8

Organic Chemistry
Chemistry in Daily LifeWhich of the following atoms contains only three
valence electrons?
Li
F
B
N
Ne

Organic Chemistry
Chemistry in Daily LifeHow many orbitals are in the d sublevel?
How many electrons can the d-orbitals hold?
How many orbitals are in the f sublevel?
How many electrons can the f-orbitals hold?

Organic Chemistry
Chemistry in Daily LifeWhich of these substances will display an incomplete octet in its Lewis structure?
Multiple Choice
CO2
Cl2
ICI
NO
S02

Organic Chemistry
Chemistry in Daily LifeConservation of Mass:Question 3
Which equation is a balanced chemical
equation?
Select one:
2NaOH+CaBr₂ → Ca(OH)₂ + NaBr
CaCO,→ CaO+2CO,
N+ Cl₂
T NCI
2A1Cl3 + 3Mg 3MgCl₂ + 2A1

Organic Chemistry
Chemistry in Daily LifeWhy is the following statement true or false?
When a match burns, the match goes away, and
thus some matter is destroyed.
False: The mass of ash is the same than the match it
came from.
True: This chemical reaction destroys matter.
True: Matter is consumed by the flame.
False: The atoms are not destroyed, they are only
rearranged.

Organic Chemistry
Chemistry in Daily LifeA. An element with the valence electron configuration 3s²3p¹ would form a monatomic ion with a charge of 1+
In order to form this ion, the element will lose 1 electron(s) from/into the p ✓subshell(s).
B. An element with the valence electron configuration 3s²3p5 would form a monatomic
In order to form this ion, the element will gain 1 electron(s) from/into the p
An error has been detected in your answer. Check for typos,
miscalculations etc. before submitting your answer.
Submit Answer
Retry Entire Group
9 more group attempts remaining
S
P
d
f
s+p
s+d
p+d

Organic Chemistry
Chemistry in Daily LifeWhen opening a bottle of perfume, the first scent one can smell is:
the Light weight, head note molecules.
all Three classes of molecules at once.
the Medium weight, heart note molecules.
the Heavy weight, bass note molecules.

Organic Chemistry
Chemistry in Daily LifeThe following unbalanced equation shows how hydrogen and oxygen combine to form water.
2H₂ + O2 H₂O
When the equation is balanced, the coefficient for water (H₂O) will be
4.
0
1
2

Organic Chemistry
Chemistry in Daily LifeLead(II) nitrate and ammonium iodide react to form lead(II) iodide and ammonium nitrate according to the reaction
Pb(NO3)₂(aq) + 2NH4I(aq) → Pbl₂ (s) + 2NH4NO3(aq)
What volume of a 0.470 M NH4l solution is required to react with 239 mL of a 0.700 M Pb(NO3)2 solution?
How many moles of Pbl2 are formed from this reaction?

Organic Chemistry
Chemistry in Daily LifeWhat will be the result of trying to cook rice by boiling water at an elevation 5,000
meters above sea level compared to doing the same cooking at sea level?
The boiling point will be higher at the higher elevation and cooking times will
increase.
The boiling point will be lower at the higher elevation and cooking times will
decrease.
The boiling point will be remain the same but cooking will be quicker.
The boiling point will be higher at the higher elevation and cooking times will
decrease.
The boiling point will be lower at the higher elevation but cooking times will
increase.

Organic Chemistry
Chemistry in Daily LifeAn atom of an unknown element can lose 3 electrons to reach a full octet.
Which of the following is most likely to be that element?
Calcium with 20 electrons in 2-8-8-2 configuration.
Aluminum with 13 electrons in 2-8-3 configuration.
Sulfur with 16 electrons in 2-8-6 configuration.
Silicon with 14 electrons in 2-8-4 configuration.

Organic Chemistry
Chemistry in Daily LifeThe first ionization energy of Al is less than that of Mg.
Select all of the following statements that are true AND describe factors contributing to the lower first ionization energy of Al as compared to Mg.
Al has no aligned spins in a partially filled 3p subshell
Al has a filled 3s subshell
Mg has a filled 3s subshell
Mg has no aligned spins in a partially filled 3s subshell

Organic Chemistry
Chemistry in Daily LifeWhat is the maximum mass of pure gold that could be extracted from 2.5 kg of calaverite, a gold ore with the chemical formula Aute?
Be sure your answer has a unit symbol, if necessary, and is rounded to 2 significant digits.

Organic Chemistry
Chemistry in Daily LifeWith respect to deoxyribonucleotide metabolism,
dATP inactivates NDPK
ATP activates ribonucleotide reductase (RNR)
dADP inhibits synthesis of dATP
all of the above are true
none of the above are true

Organic Chemistry
Chemistry in Daily LifeAn unknown compound has the following chemical formula:
PxCl5
where x stands for a whole number.
Measurements also show that a certain sample of the unknown compound contains 5.5 mol of phosphorus and 27.56 mol of chlorine.
Write the complete chemical formula for the unknown compound.

Organic Chemistry
Chemistry in Daily LifeIn 1913, Niels Bohr proposed that electrons revolve around the nucleus of an atom in discrete orbits, each orbit corresponding to a certain specific level of energy. Which one of the following statements is a consequence of this model?
When an electron absorbs energy, it moves
a higher orbit, and when it moves to a lower orbit, it emits energy.
Electrons are able to move from one orbit to another within the same atom without any gain or loss of energy.
The atomic emission spectrum for hydrogen has only one line in the visible region.
When an electron emits energy, it moves to a higher orbit, and when it moves to a lower orbit, it absorbs energy.
A maximum of only two electrons may simultaneously occupy any orbit.
![A sample of 0.0084 mol of HCI is dissolved in water to make a 1500 mL solution. Calculate the molarity of the HCI solution, the [H3O+] and the pH. For a strong acid such as HCI, the [H3O+] is the same as the molarity of the HCI solution.
HCl(aq) + H₂O (l)→ H3O+ (aq) + Cr (aq)](https://media.kunduz.com/media/sug-question/raw/58551056-1659561614.7168345.jpeg?w=256)
Organic Chemistry
Chemistry in Daily LifeA sample of 0.0084 mol of HCI is dissolved in water to make a 1500 mL solution. Calculate the molarity of the HCI solution, the [H3O+] and the pH. For a strong acid such as HCI, the [H3O+] is the same as the molarity of the HCI solution.
HCl(aq) + H₂O (l)→ H3O+ (aq) + Cr (aq)

Organic Chemistry
Chemistry in Daily LifeWrite the full electron configuration (1s²2s², etc.) for each of the following elements.
a. magnesium, Z = 12
b. carbon, Z=6

Organic Chemistry
Chemistry in Daily LifeRemember entering vary large or very small
numbers:
• For very large numbers: example 3 x 108 enter
it as 3E8
• For very small numbers: example 6.626 x 10-34
enter it as 6.626E-34
Visible light is a form of Select an answer
Electromagnetic radiation is energy transported in
the form of Select an answer
The only type of electromagnetic radiation we can
see is Select an answer

Organic Chemistry
Chemistry in Daily LifeWhich of the following is NOT a useful tip in balancing chemical equations?
Change the formula of the compounds so that the atoms balance on the reactants and products side of the equation.
Balance polyatomic ions as single entities if they appear on both sides of the equation.
Start with all other elements other than hydrogen and oxygen.
If there are an even number of atoms on one side and an odd number of atoms on the other side begin by multiplying the odds by 2.

Organic Chemistry
Chemistry in Daily LifeMr. Bean is performing an experiment in the laboratory and the procedure calls for a 2.00 M solution of HCI. Mr. Bean has a 11.9 M solution of HCI. So Mr. Bean decides he needs to make a dilution. SO Mr. Bean dissolves 0.0420 L of the 11.9 M HCL to a total volume of 500 mL. Did this give Mr. Bean a 2.00 M solution of HCI? If not how should have Mr. Bean prepared the solution?

Organic Chemistry
Chemistry in Daily LifeLiquid octane (CH₂(CH₂) CH₂) reacts with gaseous oxygen gas (0₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). If 4.18 g of water is produced from the reaction of 8.00 g of octane and 12.9 g of oxygen gas, calculate the percent yield of water.
Round your answer to 3 significant figures.

Organic Chemistry
Chemistry in Daily LifeCalcium oxide can be used to "scrub" carbon dioxide from air.
CaO(s) + CO₂(g) → CaCO3(s)
What mass of CO₂ could be absorbed by 1.75 g of CaO?
Mass= 1.375 g CO₂
What volume would this CO₂ occupy at STP?

Organic Chemistry
Chemistry in Daily Life1.16 mol sample of oxygen gas at a temperature of 27.0 °C is found to occupy a volume of 26.6 liters. The pressure of this gas sample is _ mm Hg.

Organic Chemistry
Chemistry in Daily LifeWhat is the molarity of your group's sweet tea? (1 pt)
Given the molecular structure of sugar is C₂H₂O, write out your
need to create the molarity concentration that your group
equation for molarity is: M = (4 pts: 2 pts for work, 2 pts
calculation
has been assigned
for answer)
for how many grams of sugar you will
for 175 mL of water. Remember,
the

Organic Chemistry
Chemistry in Daily LifeA sample of oxygen gas at a pressure of 797 mm Hg and a temperature of 66 °C, occupies a volume of 12.6 liters. If the gas is cooled at constant pressure to a temperature of 28 °C, the volume of the gas sample will be _ L.

Organic Chemistry
Chemistry in Daily LifeA 9.13 gram sample of krypton gas has a volume of 854 milliliters at a pressure of 3.39 atm. The temperature of the Kr gas sample is °C.

Organic Chemistry
Chemistry in Daily Life1.38 mol sample of helium gas at a temperature of 27.0 °C is found to occupy a volume of 26.7 liters. The pressure of this gas sample is

Organic Chemistry
Chemistry in Daily LifeThe concept of determining which reactant is limiting and which is in excess is akin to determining the number of sandwiches that can be made from a set number of ingredients.
Assuming that a cheese sandwich consists of 2 slices of bread and 3 slices of cheese, determine the number of whole cheese sandwiches that can be prepared from 44 slices of bread and 63 slices of cheese.
number of sandwiches:

Organic Chemistry
Chemistry in Daily LifeFor the following reaction, 47.2 grams of potassium hydrogen sulfate are allowed to react with 17.5 grams of potassium hydroxide.
potassium hydrogen sulfate (aq) + potassium hydroxide (aq) ---> potassium sulfate (aq) + water (l)
What is the maximum amount of potassium sulfate that can be formed?
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete?

Organic Chemistry
Chemistry in Daily LifeAmmonium perchlorate (NH4ClO4) is the solid rocket fuel used by the U.S. Space Shuttle. It reacts with itself to produce nitrogen gas (N₂), chlorine gas (Cl₂), oxygen gas (O₂), water (H₂O), and a great deal of energy. What mass of oxygen gas is produced by the reaction of 2.37 g of ammonium perchlorate?
Organic Chemistry
Chemistry in Daily LifeAs you increase the temperature of an enzyme-catalyzed reaction, the rate of the reaction initially
increases. It then reaches a maximum rate and finally dramatically declines. Keeping in mind that
enzymes are proteins, how do you explain these changes in reaction rates? Select the single best answer.
At first the reaction rate increases because the temperature increase causes the enzyme to
coagulate. At some point the rate of reaction dramatically decreases because the enzyme and
substrate collisions increase.
At first the rate of reaction increases because the temperature increase causes a decrease in
the number of collisions between the enzyme and the substrate. At some point the rate of
reaction dramatically decreases because the enzyme denatures due to the high temperature.
At first the reaction rate increases because the temperature increase causes the enzyme to
denature. At some point the rate of reaction dramatically decreases because the enzyme and
substrate collisions increase.
At first the rate of reaction increases because the temperature increase causes an increase in
the number of collisions between the enzyme and the substrate. At some point the rate of
reaction dramatically decreases because the enzyme denatures due to the high temperature.

Organic Chemistry
Chemistry in Daily LifeA lead fishing sinker is immersed in a solution of Mg2+ and Cu²+ cations.
Which of the two cations will the lead metal react with?
Mg2+
Cu²+
Write the balanced reaction that will occur. Be sure to include the physical states of all species.
Balanced equation:

Organic Chemistry
Chemistry in Daily LifeSolve each of the following. Remember to follow rules of rounding and significant figures
in your calculations.
In the complete reaction of 22.99 g of sodium with 35. 45g of chloride, what mass
of sodium chloride is formed?

Organic Chemistry
Chemistry in Daily LifeSome of the lipid groups appear similar, but changing a few components significantly alters their biological properties.
Identify the similarities and differences between glycerophospholipid and sphingolipid structures.
Drag the appropriate items to their respective bins.
phosphate group glycerol fatty acid
Glycerophospholipid Sphingolipid
Reset Help
sphingosine amino alcohol
Both lipids

Organic Chemistry
Chemistry in Daily LifeSome metal oxides, such as Sc₂ O3, do not react
with pure water, but they do react when the solution
becomes either acidic or basic.
Do you expect Sc₂ O3 to react when the solution becomes acidic or when it becomes basic?
The solution should be acidic.
The solution should be basic.
The reaction will occur in both cases.
Write a balanced chemical equation to support your answer.
Express your answer as a net ionic equation. Identify all of the phases in your answer.

Organic Chemistry
Chemistry in Daily LifeSolid potassium reacts with chlorine gas to produce solid potassium chloride. Write a balanced chemical equation for this reaction.

Organic Chemistry
Chemistry in Daily LifeCopper (Cu) is a metal that is commonly used for electrical wiring. Determine both the number of moles of atoms and the number of atoms in a 1.20 g sample of copper.
How many Cu atoms are in the 0.0189 mol sample?

Organic Chemistry
Chemistry in Daily LifeConsider the following reaction:
2 Al +3 CuCl₂ →3 Cu + 2 AlCl3
Assuming 73.55 g of Al are reacted with 21.83 g of copper Il chloride, how much of the excess
reactant is left behind (unused)?
Identify the limiting reactant:
CuCl2
AI

Organic Chemistry
Chemistry in Daily LifeAn organic acid is composed of carbon (48.64%), hydrogen (8.18%), and oxygen (43.20%). Its molar mass is 74.08 g/mol. Determine the molecular formula of the compound.

Organic Chemistry
Chemistry in Daily LifeHow many ATOMS of nitrogen are present in 3.94 grams of dinitrogen tetroxide ?
How many GRAMS of oxygen are present in 4.10×10²2 molecules of dinitrogen tetroxide ?

Organic Chemistry
Chemistry in Daily LifeWhich of the following types of map would best show the three
dimensions of Earth's surface?
road map
topographic map
weather map
tectonic map

Organic Chemistry
Chemistry in Daily LifeAn aqueous solution of potassium chloride is mixed with an aqueous solution of sodi
Identify the solid in the balanced equation.
a. NaCl
b. KCI
c. There is no solid formed when the two solutions are mixed.
d. NaNO3
e. KNO3