Chemistry in Daily Life Questions and Answers

Organic Chemistry
Chemistry in Daily LifeAt 448°C, the equilibrium constant for the reaction
is 50.5. What concentration of
12 would be found in an equilibrium mixture in which the concentrations of
H₂ were 0.450 M and 0.045 M, respectively?

Organic Chemistry
Chemistry in Daily LifeDoes a reaction occur when aqueous solutions of chromium(II) sulfate and barium chloride are combined?
If a reaction does occur, write the net ionic equation.
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Be sure to specify states such as (aq) or (s).

Organic Chemistry
Chemistry in Daily LifeMany chromate (CrO4) salts are insoluble, and most have brilliant colors that have led to their being used as pigments. Choose the correct net ionic equation for the reaction of Co³+ with a chromate ion.
Co³+ (aq) + CrO4 (aq) → CoC-O4(s)
2Co³+ (aq) + 3CrO4 (aq) → Co₂ (CrO4)3 (8)
2Co³+ (aq) + CrO4 (aq) → Co₂ (C-04)3(S)
2Co³+ (aq) + 3CrO4 (aq) → Co₂ (CrO4)3 (29)

Organic Chemistry
Chemistry in Daily LifeConsider the following compounds. Which is insoluble?
Fel2
PbCl₂
LiBr
AlBr3
All of these
None of these
Organic Chemistry
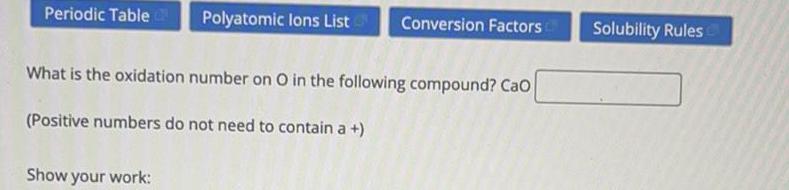
Chemistry in Daily LifeWhat is the oxidation number on O in the following compound? Cao
(Positive numbers do not need to contain a +)
Solubility Rules

Organic Chemistry
Chemistry in Daily LifeBalance the following equation and predict the states:
• Remeber to enter 1's for balancing
• States of matter options: s, I, aq, g

Organic Chemistry
Chemistry in Daily LifeA cylindrical piece weighs 20 gram. When it is dropped into a graduated cylinder, the volume of water rises from 50.0 ml to 55.5 ml.
What is the volume used to find density?
a. 105.5 ml
b. 55.5 ml
c. 50 ml
d. 5.5 ml

Organic Chemistry
Chemistry in Daily LifeAmmonia can be produced by reacting nitrogen gas with hydrogen gas.
N2 + 3H2 → 2NH3
If 1.40 g of nitrogen and 0.400 g of hydrogen are used, which reactant is in excess and how much remains? How much ammonia can be produced?

Organic Chemistry
Chemistry in Daily LifeWhat does bolded text mean in the sentence on page 176
"They often had to hold their breath."
A) it was necessary to do it
B) they were ordered to do it
C) they were not allowed to do it
D) they were expected to do it


Organic Chemistry
Chemistry in Daily LifeWrite the balanced equation for this reaction (in lowest multiple integers).

Organic Chemistry
Chemistry in Daily LifePure silicon, which is needed in the manufacturing of electronic components, may be prepared by heating silicon dioxide (sand) with carbon at high temperatures, releasing carbon monoxide gas. Choose the unbalanced
chemical equation for this process.
SiO₂ (s) + C(s) → Si(s) + CO(g)
SiO2 (s) + CO(s) → Si(s) + CO₂(g)
SiO2 (s) + CO2 (s) → Si(s) + CO(g) + O₂(g)
SiO2 (s) + C(s) + O₂(g) → SiO₂ (s) + CO(g)

Organic Chemistry
Chemistry in Daily LifeA gas cylinder contains exactly 15 moles of oxygen gas (O₂). How many molecules of oxygen
are in the cylinder?
4.01 x 1022 molecules
6.02 x 1023 molecules
9.03 x 1024 molecules
2.89 x 1026 molecules

Organic Chemistry
Chemistry in Daily LifeA medical lab is testing a new anticancer drug on cancer cells. The drug stock solution concentration is 1.5 x 10-9M, and 4.00 mL of this solution will be delivered to a dish containing 2.0 x 105 cancer cells in 5.00 mL of aqueous fluid.
What is the ratio of drug molecules to the number of cancer cells in the dish?

Organic Chemistry
Chemistry in Daily LifeA student performing the experiment "Titration of Acetic Acid in Vinegar" got mixed up where to put the solutions. He put the vinegar in the titrator, and the measured amount of NaOH solution in the beaker. He added the phenolphthalein in the beaker containing the NaOH. If he runs the titration with this set-up, what do you expect to happen to his experiment?
The solution in the beaker will initially be colorless, and the color changes from colorless to pink at the equivalence point.
The experiment will not work because it is not possible to predict the color change at the equivalence point.
The experiment will not work because there will be no color change at the equivalence point. The solution in the beaker will initially be pink, and the color changes from pink to colorless at the equivalence point.

Organic Chemistry
Chemistry in Daily LifeWhen the ideal gas law is arranged as shown below, what property of the gas is being solved for (represented by the X)?
Moles
Pressure
Temperature
Volume

Organic Chemistry
Chemistry in Daily LifeA 0.698 mol sample of Xe gas is confined in a 17.4 liter container at 30.6 °C.
If the temperature of the gas sample is decreased to 11.3 °C, holding the volume constant, the pressure will decrease because: Choose all that apply.
With lower average speeds, on average the molecules hit the walls of the container with less force.
At lower temperatures molecules have lower average speeds.
As the number of molecule-wall collisions increases, the force per collision decreases.
With higher average speeds, the molecules hit the walls of the container more often.
None of the Above

Organic Chemistry
Chemistry in Daily LifeA 0.856 mol sample of Kr gas is confined in a 21.9 liter container at 39.2 °C. If the volume of the gas sample is increased to 43.9 L holding the temperature constant, the average force per molecule-wall collision will
not enough information to answer the question
increase
remain the same
decrease

Organic Chemistry
Chemistry in Daily LifeDiazirines (H₂CN2) is a class of reactive organic chemical that, when irradiated with
ultraviolet light, will form "carbene." Give a plausible Lewis structure of H₂CNN.

Organic Chemistry
Chemistry in Daily LifeA 2 L vessel contains 4 g of Helium and 4 g of H₂ gas at 27°C. After sometime, 50% of the gas having higher average speed is removed. The percentage reduction in total pressure if temperature remains constant is equal to __

Organic Chemistry
Chemistry in Daily LifeAmong PbS, CdS, As₂S3, CuS, HgS, Ag₂S, NiS, COS, Bi₂S3, SnS₂, ZnS
a is number of black colour sulphide
b is number of white colour sulphide
c is number of yellow colour sulphide
a²-b/c
Find the value of

Organic Chemistry
Chemistry in Daily LifeWhen a single compound breaks up into two or more components, it is called a______reaction
► View Available Hint(s)
decomposition
combustion
combination
single replacement

Organic Chemistry
Chemistry in Daily LifeWrite a balanced equation for the combination reaction described, using the smallest possible integer coefficients.
When hydrogen combines with iodine, hydrogen iodide is formed.

Organic Chemistry
Chemistry in Daily LifeWhen 7.70 g of hydrocarbon (molar mas = 341.7 g/mol) is burned in a bomb calorimeter, the calorimeter increases in temperature by 2.32°C. If the heat capacity of the bomb calorimeter is 1.356 kJ/°C, what is the heat of combustion for the hydrocarbon, in kJ/mol?

Organic Chemistry
Chemistry in Daily LifeWhat should be the H-NMR multiplicity for a proton (type A) with 3 different protons (types B, C, and D) in its adjacency?
quartet
triplet
doublet of a triplet
doublet of a doublet

Organic Chemistry
Chemistry in Daily LifeFour Fe²+ ions are key components of hemoglobin, the protein that transports oxygen in the blood. If you assume that these ions are 4Fe²+, how many
protons and neutrons are present in each nucleus, and how many electrons are present in each ion?
protons
neutrons
electrons

Organic Chemistry
Chemistry in Daily LifeWhat is the name of the compound with the formula NH₂HCO3 ?
What is the name of the compound with the formula NH NO₂ ?
What is the name of the compound with the formula (NH4)3PO4?

Organic Chemistry
Chemistry in Daily Life1) (solution/solvent/solute) Use the correct word in the following sentence:
dissolved in
makes up a
2) What is the difference between a strong electrolyte and a weak electrolyte?
saturated/supersaturated/unsaturated?
3) As the temperature of the solution (increases/decreases)
solid in that liquid (increases/decreases)
As the temperature of solution (increases/decreases), _the solubility of a
that liquid (increases/decreases)
the solubility of a gas in

Organic Chemistry
Chemistry in Daily LifeA cube of aluminum at 15.4 °C is submerged into water that is 98.2 °C. In this
situation, heat will be transferred from the aluminum to the water.
True
False

Organic Chemistry
Chemistry in Daily LifeAn expandable container contains 1.25 grams of helium. What is its volume (in L)
when it has a pressure of 0.955 atm and a temperature of 22°C?
Report your answer to the correct number of significant figures. Show your work
here or on a separate sheet of paper to receive full credit for your answer.

Organic Chemistry
Chemistry in Daily LifeWhat does the term "dry" gas pressure refer to when a gas sample is collected over
water?
Select the correct answer below:
Atmospheric pressure
Vapor pressure of water
Total pressure of the pure gas plus the water vapor pressure
Pressure of the pure gas

Organic Chemistry
Chemistry in Daily LifeWhich does NOT influence the bond dipole moment?
Select the correct answer below:
The electronegativity difference between the atoms in the bond.
The magnitude of the partial charges on the atoms in the bond.
The distance between the atoms in the bond.
The bond dissociation energy of the bond.

Organic Chemistry
Chemistry in Daily Life16.Indicate whether each of the following statements describes primary, secondary,
tertiary, or quaternary protein structure:
a. Hydrophobic R groups seeking a nonpolar environment move toward the inside of
the folded protein.
b. An active protein contains four tertiary subunits.
c. Valine replaces glutamate in the beta-chain.
d. Protein chains of collagen form a triple helix.

Organic Chemistry
Chemistry in Daily LifeHow many milliliters of a 5.2 M NaCl solution would be needed to prepare each solution?
a. 15 mL of a 0.17 M solution:
b. 350 mL of a 0.039 M solution:
mL
mL

Organic Chemistry
Chemistry in Daily LifeA chemist placed a 10.00 mL sample of HCI in a flask. The HCI was titrated with 0.100
M NaOH. The titration required 22.08 mL of NaOH to reach the equivalence point.
What is the molarity of the HCI sample?
0.0221 M
0.00221 M
0.0453 M
0.221 M
Organic Chemistry
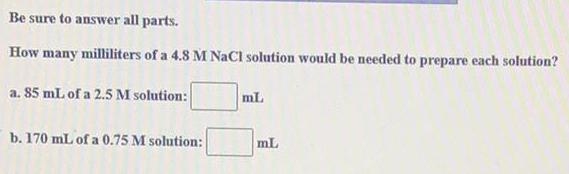
Chemistry in Daily LifeBe sure to answer all parts.
How many milliliters of a 4.8 M NaCl solution would be needed to prepare each solution?
a. 85 mL of a 2.5 M solution:
b. 170 mL of a 0.75 M solution:
mL
mL

Organic Chemistry
Chemistry in Daily LifeA cough medicine contains 0.20% (w/v) dextromethorphan, a cough suppressant, and 2.5% (w/v)
guaifenisin, an expectorant. How many milligrams of each drug would you obtain from 2.0 tsp of cough
syrup? (1 tsp = 4.93 mL)
mg dextromethorphan
mg guaifenisin

Organic Chemistry
Chemistry in Daily LifeAutomobile air bags use the decomposition of sodium azide as their
source of gas for rapid inflation:
2NaN3 (s)→ 2Na (s) + 3N₂ (g).
What mass (g) of NaN3 is required to provide 40.0 L of N₂ at 25.0 °C and
890 torr?

Organic Chemistry
Chemistry in Daily LifeWhat are the correct units of molarity.
mol/L
g/L
L/mol
g/mol
![Calculate the value of [OH-] from the given [H3O+] and label the solution as acidic or basic.
a. 4.6 × 10-3 M
)
b. 1.7 × 10-12 M](https://media.kunduz.com/media/sug-question/raw/52884476-1658982634.7046776.jpeg?w=256)
Organic Chemistry
Chemistry in Daily LifeCalculate the value of [OH-] from the given [H3O+] and label the solution as acidic or basic.
a. 4.6 × 10-3 M
)
b. 1.7 × 10-12 M

Organic Chemistry
Chemistry in Daily LifeBe sure to answer all parts.
Calculate the formula weight and molar mass of the following biologically active compound.
C16H16CINO2S (Plavix), a drug used to treat coronary artery disease
amu,
g/mol

Organic Chemistry
Chemistry in Daily LifeBe sure to answer all parts.
How many moles of NaCl are contained in each volume of aqueous NaCl solution?
a. 7.7 L of a 2.9 M solution
mol NaCl
b. 82 mL of a 5.4 M solution
mol NaCl

Organic Chemistry
Chemistry in Daily LifeWhat is the weight/volume percent concentration using the given amount of solute and total volume of
solution?
50.0 g of LiCl in 850 mL of solution:
% (w/v) LICI

Organic Chemistry
Chemistry in Daily LifeWhich represents the greatest mass of sulfur?
1 gram of sulfur
0.5 mole of sulfur
1 molecule of sulfur
6.02 x 1023 atoms of sulfur
How many moles are in 1.204 x 1024 molecules of CO?
2 moles
6.02 moles
5 grams
1.2 moles

Organic Chemistry
Chemistry in Daily LifeHow hot must the air in a balloon be heated if initially it has a volume of 700. L at 20°C and the final
volume must be 1,360. L? The balloon is sealed so that the pressure and number of air particles are
constant.
K

Organic Chemistry
Chemistry in Daily LifePoints 1
Identify the property of ionic compounds.
Insoluble in water.
Brittle
Low melting point.
Low boiling point.

Organic Chemistry
Chemistry in Daily LifeBe sure to answer all parts.
Calculate the number of grams contained in each of the following number of moles. Report your answer
with the appropriate number of significant figures.
a. 0.150 mol of NaCl (sodium chloride)
b. 6.20 mol of C₂H4 (ethylene)
g

Organic Chemistry
Chemistry in Daily LifeWhich of the following lists the relative strengths of the fundamental forces
from strongest to weakest?
A. Strong nuclear force, weak nuclear force, gravitational force,
electrostatic force
B. Gravitational force, strong nuclear force, electrostatic force, weak
nuclear force
C. Electrostatic force, gravitational force, weak nuclear force, strong
nuclear force
D. Strong nuclear force, electrostatic force, weak nuclear force,
gravitational force

Organic Chemistry
Chemistry in Daily LifeWhich statement describes the potential energy diagram of an exothermic
reaction?
A. The potential energy of the products is greater than the potential
energy of the reactants.
B. The activation energy of the reactants is greater than the
activation energy of the products.
C. The potential energy of the reactants is greater than the potential
energy of the products.
D. The potential energy of the products is equal to the potential
energy of the reactants.

Organic Chemistry
Chemistry in Daily LifeQuestion 2 of 25
How can an unknown ΔHreaction be determined using Hess's law?
A. The free energy of the reaction is used to determine the AH for
the reaction.
B. The unknown ΔHreaction is determined after the reaction is run in a
calorimeter.
C. The reaction is repeated at different temperatures to determine
the ΔHreaction-
D. Enthalpies from reaction steps are added to determine an
unknown AHreaction