General organic chemistry Questions and Answers

Organic Chemistry
General organic chemistryAccording to the following reaction, how many moles of bromine monochloride will be formed upon the complete reaction of 0.188 moles bromine with excess
chlorine gas?
bromine (g) + chlorine (g) ---> bromine monochloride (g)
moles bromine monochloride

Organic Chemistry
General organic chemistryThe theoretical yield of a reaction is the amount of product obtained if the limiting reactant is completely converted to product.
Consider the reaction:
2 CO(g) + O₂(g) → 2 CO₂(g)
If 9.810 g CO is mixed with 11.82 g O₂, calculate the theoretical yield (g) of CO₂ produced by the reaction.

Organic Chemistry
General organic chemistryAn iron nail rusts when exposed to oxygen.
For the following reaction, 4.26 grams of oxygen gas are mixed with excess iron. The reaction yields 12.2 grams of iron(III) oxide.
iron (s) + oxygen (g) ---> iron(III) oxide (s)
What is the theoretical yield of iron(III) oxide ?
What is the percent yield for this reaction ?

Organic Chemistry
General organic chemistryFor the following reaction, 11.9 grams of nitrogen monoxide are allowed to react with 11.3 grams of oxygen gas.
nitrogen monoxide(g) + oxygen(g) ---> nitrogen dioxide (g)
What is the maximum mass of nitrogen dioxide that can be formed?
What is the FORMULA for the limiting reagent?
What mass of the excess reagent remains after the reaction is complete?

Organic Chemistry
General organic chemistryFor the following reaction, 11.2 grams of glucose (C6H12O6) are allowed to react with 15.7 grams of oxygen gas.
glucose (C6H12O6)(s) + oxygen(g) → carbon dioxide(g) + water(l)
What is the maximum amount of carbon dioxide that can be formed?
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete?

Organic Chemistry
General organic chemistryWrite the formula for the following compounds:
sodium fluoride; nitrous acid; carbon trioxide; calcium hydroxide; nitrogen tribromide; magnesium chloride; dinitrogen heptachloride; aluminum sulfate; sulfuric acid lithium sulfate; silver nitrate; calcium sulfate; iron (III) chloride disulfur tetrafluoride: mercury (II) nitrate: hydrochloric acid carbon tetrachloride; lead (IV) nitrate; magnesium iodide sodium nitride: trihydrogen monophosphide ; sodium carbonate; copper (II) sulfate; magnesium hydroxide; nitrogen pentoxide; barium nitrate

Organic Chemistry
General organic chemistryIf sodium peroxide is added to water, elemental oxygen gas is generated, consider this unbalanced equation:
Na₂O₂ (s) + H₂O(l)→ NaOH(aq) + O₂(g)
Suppose 4.70 g of sodium peroxide is added to a large excess of water. What mass of oxygen gas will be produced?
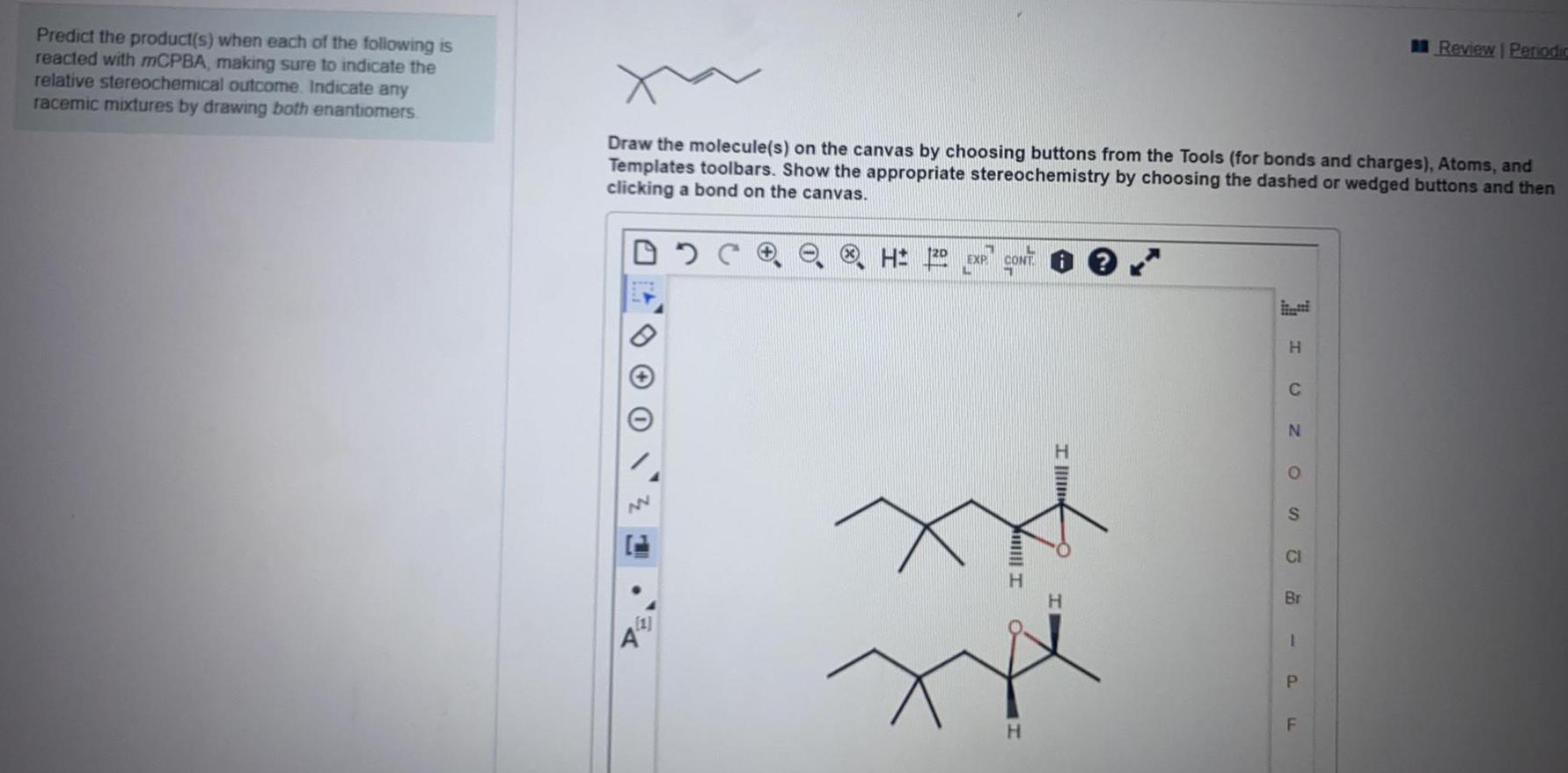
Organic Chemistry
General organic chemistryPredict the product(s) when each of the following is reacted with mCPBA, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers. Draw the molecule(s) on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars. Show the appropriate stereochemistry by choosing the dashed or wedged buttons and then clicking a bond on the canvas.

Organic Chemistry
General organic chemistryFor the following reaction, 33.0 grams of bromine are allowed to react with 13.1 grams of chlorine gas.
bromine(g) + chlorine(g) → bromine monochloride (g)
What is the maximum mass of bromine monochloride that can be formed?
What is the FORMULA for the limiting reagent?
What mass of the excess reagent remains after the reaction is complete?

Organic Chemistry
General organic chemistryFor the following reaction, 12.0 grams of sodium are allowed to react with 6.65 grams of water.
sodium(s) + water (l) ---> sodium hydroxide (aq) + hydrogen(g)
What is the maximum amount of sodium hydroxide that can be formed?
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete?

Organic Chemistry
General organic chemistryFor the following reaction, 6.18 grams of potassium hydroxide are mixed with excess potassium hydrogen sulfate. The reaction yields 17.0 grams of potassium sulfate.
potassium hydrogen sulfate (aq) + potassium hydroxide (aq) ---> potassium sulfate (aq) + water(l)
What is the theoretical yield of potassium sulfate?
What is the percent yield of potassium sulfate?

Organic Chemistry
General organic chemistrySilicon carbide, SiC, is one of the hardest materials known. Surpassed in hardness only by diamond, it is sometimes known commercially as carborundum. Silicon carbide is used primarily as an abrasive for sandpaper and is manufactured by heating common sand (sillcon dioxide, SiO₂) with carbon in a furnace.
SiO₂ (s) + C(s) → CO(g) + SiC(s)
What mass of silicon carbide should result when 3.4 kg of pure sand is heated with an excess of carbon?

Organic Chemistry
General organic chemistryUrea, chemical formula (NH₂)₂CO, is used for fertilizer and many other things. Calculate the number
of N, C, O, and H atoms in 1.68 x 104 g of urea. Enter your answers in scientific notation.

Organic Chemistry
General organic chemistryFor the following reaction, 23.9 grams of sulfur dioxide are allowed to react with 9.27 grams of oxygen gas.
sulfur dioxide (g) + oxygen (g) ---> sulfur trioxide (g)
What is the maximum amount of sulfur trioxide that can be formed?
What is the FORMULA for the limiting reagent?
What amount of the excess reagent remains after the reaction is complete? 46.35

Organic Chemistry
General organic chemistryName and Describe the interaction between polar molecules?

Organic Chemistry
General organic chemistryDetermine the number of moles of NaOH neutralized by 395 mL 0.610 M HNO3.

Organic Chemistry
General organic chemistryA pure sample of (R)-2-butanol has a specific rotation
of -13.5°. A sample of 2-butanol was found to have an
observed rotation of +6.75. What are the percentages
of (R)- and (S)-2-butanol in the sample (10 pts)?

Organic Chemistry
General organic chemistryDraw the molecular orbital (MO) electron diagram for the N₂ molecule.
Be sure your diagram contains all of the electrons in the molecule, including any core electrons.

Organic Chemistry
General organic chemistryWhat is another name for nonpolar molecules? Polar molecules?

Organic Chemistry
General organic chemistryDraw the skeletal ("line") structure of a secondary alcohol with 6 carbon atoms, 1 oxygen atom, and no double or triple bonds.

Organic Chemistry
General organic chemistryPure (5R.6R)-5,6-di-tert-butyldecane has a specific rotation of +53. A student
tried to synthesize the compound in lab and found that her sample had a
specific rotation of -33.
a. What would be the specific rotation for an enantiomerically pure sam
of (55.6S)-5-6-di-tert-butyldecane?
b. What is the % of the solution? Which enantiomer is in excess?
c. What is the specific rotation of (5S,6R)-5,6-di-tert-butyldecane?

Organic Chemistry
General organic chemistryPure (5R,6R)-5,6-di-tert-butyldecane has a specific rotation of +53. A student tried to synthesize the compound in lab and found that her sample had a specific rotation of -33.
a. What would be the specific rotation for an enantiomerically pure sample of (5S,6S)-5,6-di-tert-butyldecane?
b. What is the %ee of the solution? Which enantiomer is in excess?
c. What is the specific rotation of (5S,6R)-5,6-di-tert-butyldecane?

Organic Chemistry
General organic chemistryGreen plants use light from the Sun to drive photosynthesis. Photosynthesis is a chemical reaction in which water (H₂O) and carbon dioxide (CO₂) chemically react to form the simple sugar glucose (C6H₁2O6) and oxygen gas (O₂). What mass of oxygen gas is produced by the reaction of 9.03 g of carbon dioxide? Round your answer to 3 significant digits.

Organic Chemistry
General organic chemistryUsing the equation Zeff = Z-S and assuming that the core electrons contribute 1.00 and valence electrons contribute nothing to the screening constant, S, calculate Zeff for these two ions.
Express your answers as integers. Enter your answers numerically separated by a comma.
Repeat this calculation using Slater's rules to estimate the screening constant, S, and calculate Zeff for these two ions.
Express your answers using two decimal places. Enter your answers numerically separated by a comma.

Organic Chemistry
General organic chemistryWhich of the following elements has the smallest atomic number?
sulfur, which has sixteen protons
lithium, which has three protons
scandium, which has twenty-one protons
helium, which has two protons

Organic Chemistry
General organic chemistryWhen we talk about the osmotic pressure
of a solution, what do we mean?
the temperature decrease when a
solvent freezes
water exerts a pressure in a closed
container
the partial pressure of the water
above a solution
the pressure that is needed to stop
the net flow of solvent across a
semipermeable membrane.

Organic Chemistry
General organic chemistryConcentrated sulfuric acid has a density of 1.84 g/ml.
A) What's the specific gravity of sulfuric acid?
B) Calculate the mass in grams of 1.00 liter sulfuric aci
Show your calculations for answer (B).

Organic Chemistry
General organic chemistryFor the chemical reaction
HCIO4 (aq) + NaOH(aq) → H₂O(l) + NaCIO4 (aq)
write the net ionic equation, including the phases.
net ionic equation:
Which ions are considered spectator ions for this reaction?
OH-
Na+
H+
CIO4-

Organic Chemistry
General organic chemistryOne of the alkali metals reacts with oxygen to form a solid white substance. When this substance is dissolved in water, the solution gives a positive test for hydrogen peroxide, H₂O₂.
When the solution is tested in a burner flame, a lilac-purple flame is produced. What is the likely identity of the metal?
Express your answer as a chemical symbol.
Write a balanced chemical equation for reaction of the white substance with water.
Express your answer as a chemical equation. Identify all of the phases in your answer.

Organic Chemistry
General organic chemistryAqueous potassium nitrate (KNO3) and solid silver bromide are formed by the reaction of aqueous silver nitrate (AgNO3) and aqueous potassium bromide Write a balanced chemical equation for this reaction.

Organic Chemistry
General organic chemistryFor this problem, identify the limiting reagent and calculate the grams of CO₂ obtained in the reaction of 140.0 grams of C7H12O5N3 with 120.0 grams of oxygen. If 125 grams of CO₂ is actually produced, what is the % yield. The equations are not balanced.
C7H12O5N3+ O2 -> CO₂ + H₂O + NH3
What is the limiting reagent?

Organic Chemistry
General organic chemistryHydrogen gas and aqueous sodium hydroxide (NaOH) are formed by the reaction of liquid water and solid sodium.
Write a balanced chemical equation for this reaction.

Organic Chemistry
General organic chemistryWhat is the proper dissociation equation for Li3N, a strong electrolyte?
a. Li3N(s) <→ Li³+(aq) + 3N-(aq)
b. Li3N(s) → Li³+(aq) + 3N-(aq)
c. Li3N(s) <→ Li+(aq) + N³-(aq)
d. Li3N(s) <→ 3Li+(aq) + 3N-(aq)
e. Li3N(s) → 3Li+(aq) + N³-(aq)

Organic Chemistry
General organic chemistryCellulose is a polysaccharide which has
A. only beta-1,4-bonds between glucose units
B. only alpha-1,4-links bonds glucose units
C. hemiacetal links joining glucose units
D. both alpha-1,4- and bonds between glucose units

Organic Chemistry
General organic chemistryIdentify the neutral element represented by this excited-state electron configuration, then write the ground-state electron configuration for that element.
excited state: 1s22s²2p⁰3s¹

Organic Chemistry
General organic chemistryDraw the skeletal ("line") structure of methylcyclopropane.

Organic Chemistry
General organic chemistryPlease provide a mechanism for the following transformation. You may use general base, B, and general acid H+ in your answer.
note: tributyl phosphine is a catalyst in this reaction

Organic Chemistry
General organic chemistrySuppose all the chlorine atoms in this molecule are replaced by hydrogen atoms:
Draw a skeletal ("line") structure of the new molecule.
Be sure you follow all the usual rules for drawing skeletal structures.

Organic Chemistry
General organic chemistryPredict the product(s) when each of the following are reacted with mCPBA, making sure to indicate the relative stereochemical outcome. Indicate any racemic mixtures by drawing both enantiomers.

Organic Chemistry
General organic chemistryWhich of the following sets of elements contain all transition elements?
Cobalt, Rhodium, Gold, and Mercury
Silicon, Germanium, lodine, and Lead
lodine, Xenon, Antimony, and Indium
Helium, Calcium, Radium, and Beryllium

Organic Chemistry
General organic chemistryWhy is the ammonium salt of lidocaine used rather than the amine?
The ammonium salt (lidocaine hydrochloride) is in water and body fluids than the amine lidocaine.
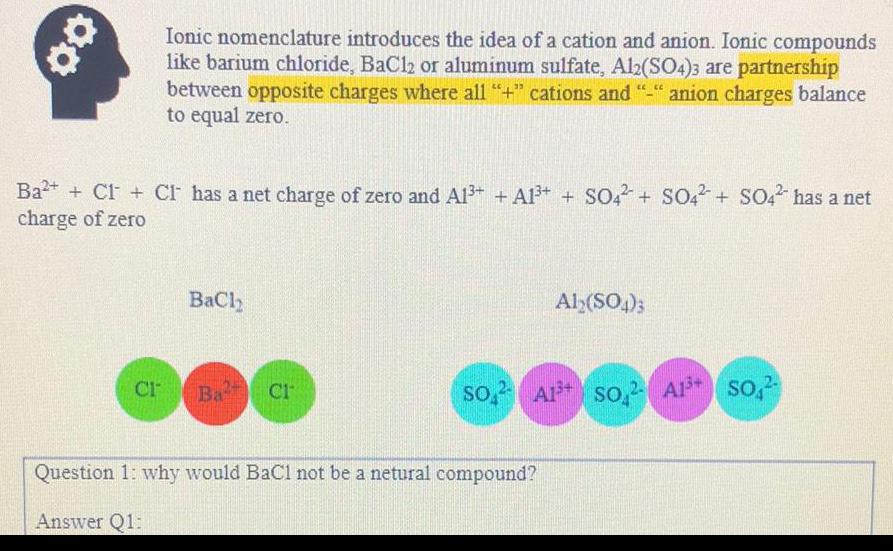
Organic Chemistry
General organic chemistryIonic nomenclature introduces the idea of a cation and anion. Ionic compounds like barium chloride, BaCl2 or aluminum sulfate, Al2(SO4)3 are partnership between opposite charges where all "+" cations and "-" anion charges balance to equal zero.
Ba²+ + CI- + Cl- has a net charge of zero and Al³+ + Al³+ + SO42- + SO4²- + SO4²- has a net charge of zero
BaCI2 Al₂(SO4)3
Question 1: why would BaCl not be a netural compound?

Organic Chemistry
General organic chemistryWhich of the following is the major product of the reaction of 1-propanol with a strong oxidizing agent?

Organic Chemistry
General organic chemistryA student weighs out 4.66 g of benzaldehyde (C6H5CHO) to react with sodium borohydride (NaBH₂) according to the following chemical equation:
C6H5CHO + NaBH4 → C6H5CH₂OH
To ensure complete consumption of benzaldehyde, 1.5 equivalents of NaBH4 are used. Calculate how many grams of NaBH, are needed.
(MW_C6H5CHO = 106.12 g/mol; MW_NaBH4 = 37.83 g/mol)

Organic Chemistry
General organic chemistryWhat is the IUPAC name of the following ether?
CH3-CH2-CH₂-CH2-O-CH3
2-methoxybutane
1-butoxymethane
butyl methyl ether
1-methoxybutane

Organic Chemistry
General organic chemistryHow many molecules of SO3 can be formed from 0.74 moles of O₂
(assuming excess SO₂) from the following UNBALANCED equation?
SO₂(g) + O₂(g) → SO3(g)
![What is the rate law for the following mechanism in terms of the overall rate constant k?
Step 1: A+B = C (fast)
Step 2: B+C → D (slow)
Express your answer in terms of k and the necessary concentrations (e.g., k* [A]^3* [D]).](https://media.kunduz.com/media/sug-question/raw/56688773-1659465675.0283806.jpeg?w=256)
Organic Chemistry
General organic chemistryWhat is the rate law for the following mechanism in terms of the overall rate constant k?
Step 1: A+B = C (fast)
Step 2: B+C → D (slow)
Express your answer in terms of k and the necessary concentrations (e.g., k* [A]^3* [D]).

Organic Chemistry
General organic chemistryA certain reaction has an activation energy of 70.0 kJ/mol and a frequency factor of A₁ = 5.00x1012 M-¹s-¹. What is the rate constant, k, of this reaction at 29.0 °C ?
Express your answer with the appropriate units. Indicate the multiplication of units explicitly either with a multiplication dot (asterisk) or a dash.

Organic Chemistry
General organic chemistryThe empirical formula for vinegar is CH₂O. Determine the molecular formula of vinegar if the molar mass is known to be about 60 g/mol.
What is the molar mass of your molecular formula, C₂H4O2? (Enter your answer to two decimal places.)

Organic Chemistry
General organic chemistryA canister of chlorine gas contains 2,270 mL under a pressure of 0.836 atm. Assuming
unchanging temperature and amount of gas, what is the pressure when the volume is decreased
to 1.00L?
a. 0.368 atm
b. 1.90 atm
C. 2.72 atm
d. 1,900 atm
e. 2,720 atm