General organic chemistry Questions and Answers


Organic Chemistry
General organic chemistryCopper (Cu) is element 29 on the periodic table. Calculate the mass, in grams, of 1.46 mol of Cu.
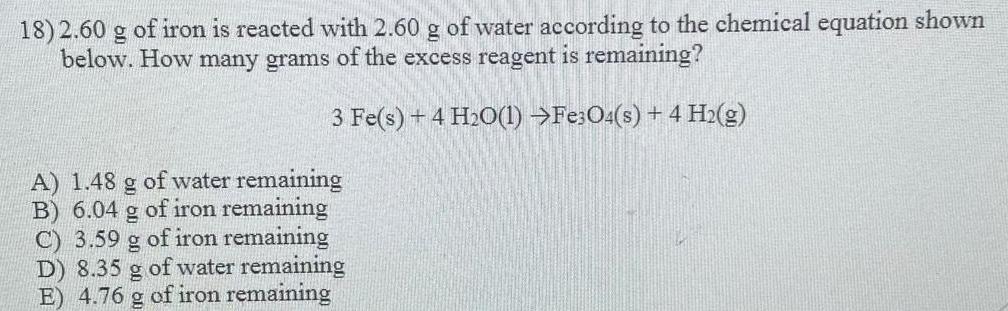
Organic Chemistry
General organic chemistry2.60 g of iron is reacted with 2.60 g of water according to the chemical equation shown
below. How many grams of the excess reagent is remaining?
3 Fe(s) + 4H₂O(l) ---> Fe3O4(s) + 4 H₂(g)
A) 1.48 g of water remaining
B) 6.04 g of iron remaining
C) 3.59 g of iron remaining
D) 8.35 g of water remaining
E) 4.76 g of iron remaining

Organic Chemistry
General organic chemistryA sample of Xe weighs 20.5 grams. Will a sample of Ca that contains the same number of atoms weigh more or less than 20.5 grams?
Calculate the mass of a sample of Ca that contains the same number of atoms.

Organic Chemistry
General organic chemistryThe following reaction steps are shown using conventional electron pushing. (a) Draw the
second product whose formation would have been rationalized with this same arrow. (b)
Use the bouncing arrow formalism to illustrate the formation of only the product shown.

Organic Chemistry
General organic chemistryWhich of the following is a FALSE statement?
a. Pressure is created by collisions between gas particles and the walls of container.
b. Atmospheric pressure is lower at higher altitudes.
C. A barometer is a device used to measure atmospheric pressure.
d. Standard temperature and pressure is 0 °C and 1 atm.
e. A small amount of the volume occupied by a gas is empty space.

Organic Chemistry
General organic chemistryWhat is the IUPAC name of this compound?
A) butanamide
B) N-methylbutanamide
C) N-dimethylbutanamide
D) N,N-dimethylbutanamide
E) dimethylbutanamide

Organic Chemistry
General organic chemistryComplete the table below for calculating the molar mass of the compound sulfur dichloride.

Organic Chemistry
General organic chemistryFind the oxidation numbers for each element in the following compound. Label positive values as "+X" and negative values as "-X" where X is the oxidation number you calculate.
Mg(MnO4)2

Organic Chemistry
General organic chemistryAt 473 K, the pressure of a sample of nitrogen is 1050 mmHg. What will the temperature be in
°C (assuming constant volume and amount of gas) if the pressure is decreased to 833 mmHg?
a. 102 °C
b. 324 °C-
C. 375 °C
d. 597 °C
e. 648 °C

Organic Chemistry
General organic chemistryYou have not correctly identified the
value of x. You know that x represents
the number of grams of sucrose. First,
calculate the number of moles of solute
in 250 mL of a 0.100 M solution. Then,
convert from moles to grams using the
molar mass of sucrose.

Organic Chemistry
General organic chemistryWhen a 0.2481-g sample of titanium metal is reacted with oxygen, the final mass of the resulting compound is 0.4139 g. Determine the empirical formula of this titanium oxide.
HOW DO WE GET THERE?
What is the mass of each element present in the compound? (Enter your answers to four significant figures.)

Organic Chemistry
General organic chemistryHow many grams of Kr are there in a sample of Kr that contains 9.27×10^23 atoms?

Organic Chemistry
General organic chemistryVinegar contains carbon, hydrogen, and oxygen with percent masses of 40.01% C, 6.70% H, and 53.29% O, respectively. Determine the empirical formula of the compound.
HOW DO WE GET THERE?
What is the mass of each element in 100.0 g of vinegar? (Enter your answers to two decimal places.)

Organic Chemistry
General organic chemistryAn organic acid is composed of carbon (62.04%), hydrogen (10.43%), and oxygen (27.55%). Its molar mass is 116.16 g/mol. Determine the molecular formula of the compound.

Organic Chemistry
General organic chemistryHow many grams of Si are there in a sample of Si that contains the same number of moles as a 107 gram sample of Co?

Organic Chemistry
General organic chemistryA compound is found to contain 55.39 % boron, 8.280 % hydrogen, and 36.33 % chlorine by mass.
What is the empirical formula for this compound?

Organic Chemistry
General organic chemistryOzone (O3) is formed in the earth's upper atmosphere by the action of solar radiation on oxygen
molecules (O2). Write a balanced equation for the formation of ozone from oxygen.
Balance the following equations:
a. Ca(OH)2+HCl-CaCl2+H2O
b. Al +O2-Al2O3
C. CH3CH3+02-CO2+H2O
d. AgNO3+MgCl2-AgCl +Mg(NO3)2
Organic Chemistry
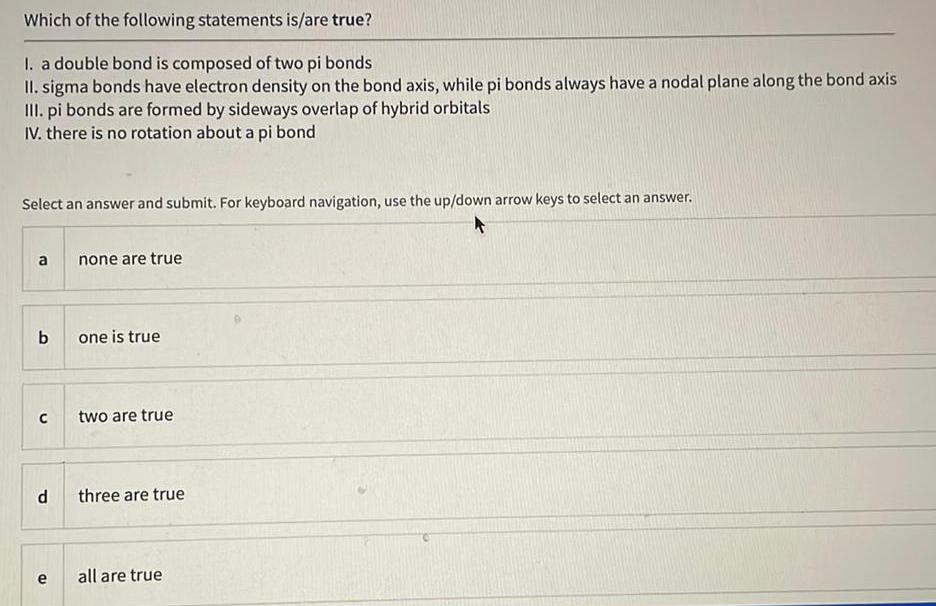
General organic chemistryWhich of the following statements is/are true?
I. a double bond is composed of two pi bonds
II. sigma bonds have electron density on the bond axis, while pi bonds always have a nodal plane along the bond axis
III. pi bonds are formed by sideways overlap of hybrid orbitals
IV. there is no rotation about a pi bond
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
a none are true
b one is true
c two are true
d three are true
e all are true

Organic Chemistry
General organic chemistryA compound 'P' containing Bromine in it, on treatment with
NH3 produces 'Q', which is solid at room temperature and is
free from Bromine atom. The solid Q contains 49.3% Carbon,
9.59% Hydrogen, 19.18% Nitrogen and 21.93% Oxygen. 'Q' on
reaction with Br₂ and Ca(OH)₂ gives a product 'R'. The
product 'R' can give carbylamine test and give ethene on
reaction with excess of CH3 - I followed by heating with
moist Ag₂O.

Organic Chemistry
General organic chemistryA compound is found to contain 37.47 % carbon, 12.61 % hydrogen, and 49.92 % oxygen by mass.
To answer the question, enter the elements in the order presented above.
QUESTION 1:
The empirical formula for this compound is
QUESTION 2:
The molar mass for this compound is 32.05 g/mol.
The molecular formula for this compound is

Organic Chemistry
General organic chemistryA compound was analyzed and was found to contain the following percentages of the elements by mass: sulfur, 94.09%; hydrogen, 5.91%. Determine the empirical formula of the compound.
Empirical formula:

Organic Chemistry
General organic chemistryA compound having an approximate molar mass of 165.0-170.0 g has the following percentage composition by mass:
carbon, 42.86 %
hydrogen, 3.598 %
nitrogen, 25.00 %
oxygen, 28.54 %
Determine the empirical and molecular formulas of the compound.
(Enter the elements in the order: C, H, N, O.)
Empirical formula:
Molecular formula:

Organic Chemistry
General organic chemistryA compound is found to contain 26.73 % phosphorus, 12.09 % nitrogen', and 61.18 % chlorine by mass.
To answer the question, enter the elements in the order presented above.
QUESTION 1:
The empirical formula for this compound is____.
QUESTION 2:
The molar mass for this compound is 115.9 g/mol.
The molecular formula for this compound is_____.

Organic Chemistry
General organic chemistryOne mole of H₂O and one mole of CO are taken in a 10 litre vessel and heated to 725 K. At equilibrium, 40 percent of water (by mass) reacts with carbon monoxide according to the equation:
H₂O(g) + CO(g) ⇒ H2(g) + CO2(g)
Calculate the equilibrium constant for the reaction.

Organic Chemistry
General organic chemistryA 5.746 gram sample of chromium is heated in the presence of excess fluorine. A metal fluoride is formed with a mass of 12.04 g. Determine the empirical formula of the metal fluoride
Enter the elements in the order Cr, F
empirical formula =
Organic Chemistry
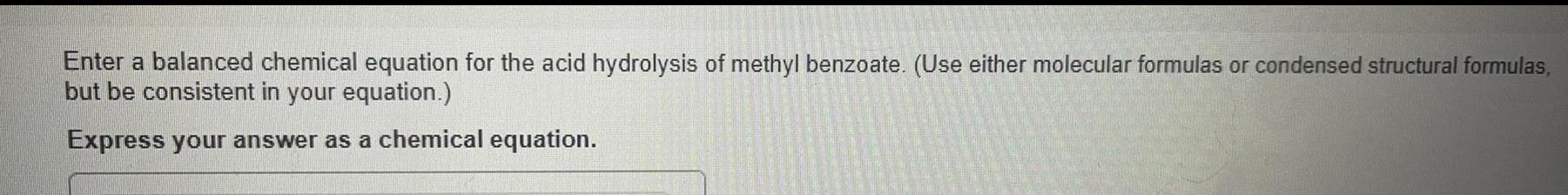
General organic chemistryEnter a balanced chemical equation for the acid hydrolysis of methyl benzoate. (Use either molecular formulas or condensed structural formulas,but be consistent in your equation.) Express your answer as a chemical equation.

Organic Chemistry
General organic chemistryA 42.96 gram sample of cobalt is heated in the presence of excess sulfur. A metal sulfide is formed with a mass of 78.03 g. Determine the empirical formula of the metal sulfide.

Organic Chemistry
General organic chemistryCalculate the percentage composition for CBr4.
Mass percentage of carbon =
Mass percentage of bromine =

Organic Chemistry
General organic chemistryDetermine the concentrations of MgCl₂, Mg2+, and CI in a solution prepared by dissolving 2.43 x 10 g MgCl₂ in 2,25 L of water. Express all three concentrations in molarity. Additionally, express the concentrations of the ionic species in parts per million (ppm).

Organic Chemistry
General organic chemistryHow many ATOMS of carbon are present in 5.28 grams of carbon tetrabromide ? 9.58x10^21 2. How many GRAMS of bromine are present in 8.91x1022 molecules of carbon tetrabromide ?

Organic Chemistry
General organic chemistryParts per million (ppm) is a common way to express small concentrations of a solute in water. A sample of tap water that is 25 ppm Cl contains 25 grams of CI for every 1,000,000 grams of water. Which units are numerically equal to ppm for dilute aqueous solutions?
g/L
mg/L.
cg/L.
µg/L
ng/L
Organic Chemistry
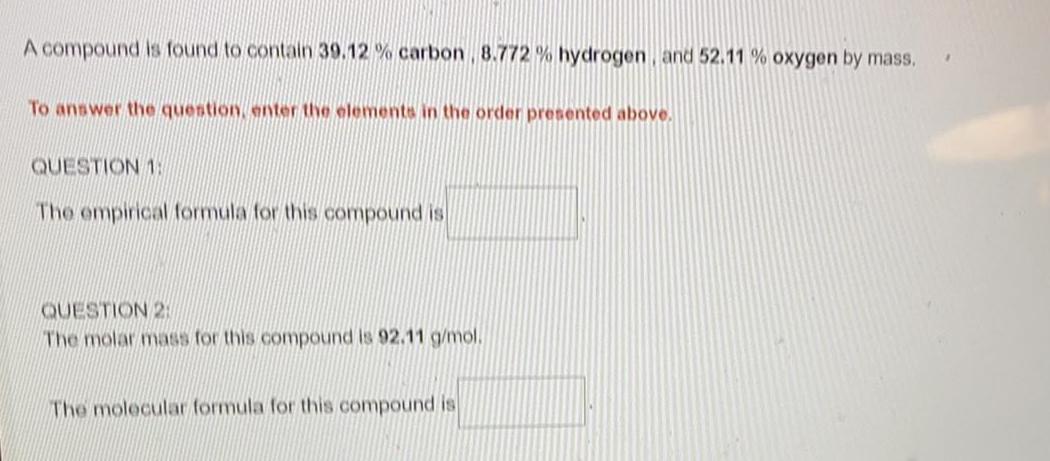
General organic chemistryA compound is found to contain 39.12% carbon, 8.772 % hydrogen, and 52.11 % oxygen by mass.
The empirical formula for this compound is
The molar mass for this compound is 92.11 g/mol.
The molecular formula for this compound is

Organic Chemistry
General organic chemistryCalculate the mass in grams for each of the following samples.
a. 1.08 moles of aluminum chloride
b. 3.62 moles of sodium hydrogen carbonate
c. 4.21 millimoles of hydrogen bromide (1 millimole = 1/1000 mole)

Organic Chemistry
General organic chemistryWhat would you multiply "atoms of cesium" by to get the units "moles of cesium"?

Organic Chemistry
General organic chemistryWhat would you multiply "moles of cesium" by to get the units "grams of cesium"?
Drag and drop your selection from the following list to complete the answer:

Organic Chemistry
General organic chemistryA cat with a mass of 5 kg is sitting on a skateboard. A rat with a mass of 0.5 kg is sitting on an identical skateboard. 2. If you apply the same force to push each skateboard, will the cat or the rat accelerate the fastest? Explain.

Organic Chemistry
General organic chemistryWhich of the following actions would cause the rate of the reaction below to decrease?
CuSO4 + Mg MgSO4 + Cu
a. Heating the reaction.
b. Adding magnesium.
c. Removing copper.
d. Adding a catalyst.
e. Cooling the reaction.

Organic Chemistry
General organic chemistryIf 17.4 grams of hydrochloric acid (HCI) reacted, how many grams of hydrogen (H₂) were produced? NOTE: HCI has a molar mass of 36.46g and H₂ has a molar mass of 2.02g.

Organic Chemistry
General organic chemistryThe hydrogen deficiency index (HDI) of C4H100 is, which means this compound has pi bonds or rings or combination of pi bonds and rings.
2
3
Clear selectic

Organic Chemistry
General organic chemistryA 1.5g sample of coniine (shown below) was dissolved in 10 mL of ethanol and placed in a cell with a 5.0 cm path length. The observed rotation was +1.21°. Calculate the specific rotation of coniine (2 pts). Is ethanol an acceptable solvent to use?

Organic Chemistry
General organic chemistryDraw the expanded structural formula of two water molecules and indicate how each of these forms hydrogen bonds with the molecule shown below. Use dotted lines to indicate hydrogen bonds. --- Files uploaded sideways or upside down will not be graded.


Organic Chemistry
General organic chemistryLaurediol is a compound isolated from marine algae. Designate sequentially (1,2,3,4) the stereochemistry at the numbered sites in the given drawing of Laurediol.

Organic Chemistry
General organic chemistryThe e.m.f of cell in V: H₂(g) |Buffer || Normal caloal electrode is 0.6885 V at 40°C when the barometric pressure is 725 mm of Hg. What is the pH of the solution. E = 0.28 .(write the value to the nearest integer)

Organic Chemistry
General organic chemistryHow many mL of 0.75 M H3PO4 would be required to titrate 200.0 mL of 0.50 M Ca(OH)2 to
the equivalence point? (4 points) The balanced reaction has been provided.

Organic Chemistry
General organic chemistryConsider the following reaction:
2C₂H6 + 702 4CO2 + 6H₂O
Identify what main category of reaction it is. If possible, further categorize it into all other relevant
types of reaction.
Synthesis
Decomposition
Combustion
Single Replacement
Double Replacement
Precipitation
Acid-Base
Oxidation-Reduction

Organic Chemistry
General organic chemistryConsider the following reaction:
K₂SO4 + Ba(NO3)2 → 2KNO3 + BaSO4
Identify what main category of reaction it is. If possible, further categorize it into all other relevant types of reaction.
Synthesis
Decomposition
Combustion
Single Replacement
Double Replacement
Precipitation
Acid-Base
Oxidation-Reduction
Gas Evolving


Organic Chemistry
General organic chemistryBalance the equation
Fe2S3(s)→ Fe(s) + S(s) + 69kJ
1b) What is the mole to mole ratio of Fe₂S3(s) to Fe(s)
1c.) What is molar mass of Fe2S3(s)
ld.) How many moles is 28 gram of Fe2S3(s)?
le. How many moles of Fe(s) does this produce using answers 1b and 1d.