General organic chemistry Questions and Answers

Organic Chemistry
General organic chemistryA gas sample has a pressure of 747 mmHg when the temperature is 26 °C.
Part A
What is the final temperature, in degrees Celsius, when the pressure is 780 mmHg, with no change in the volume or amount of gas? Express your answer to two significant figures and include the appropriate units.

Organic Chemistry
General organic chemistryDraw a structural formula for the following alkene: cis-2-methyl-3-hexene
▪ Consider E/Z stereochemistry of alkenes.
▪ Do not show stereochemistry in other cases.
▪ You do not have to explicitly draw H atoms.

Organic Chemistry
General organic chemistryWhich is the correct net ionic equation for the formation of calcium
oxalate kidney stones?
Ca²+ (s) + C₂04² (s)--> CaC204(aq)
Ca²+ (aq) + C₂04² (aq) -->CaCO3(s)
Cat (aq) + C2O4 (aq) -->CaC₂O4(s)
Ca2+(aq) + C₂04² (aq)-->CaC₂O4(s)

Organic Chemistry
General organic chemistryUpload the model and line formula for Benzoic acid (a benzene ring with COOH attached to one C in the ring). Benzoic acid is insoluble in water but soluble in aqueous sodium hydroxide. Explain mentioning the appropriate IMFs. Hint see canvas lecture Draw the condensed or line formula for sodium benzoate (the benzoic acid deprotonated and a sodium ion counterion) Benzene- COO- Na+

Organic Chemistry
General organic chemistryOne mole of an alkene (E) on ozonolysis gives two different compounds X and Y. X and Y are the isomers of each other and their molar mass is
equal to 58 u, each. Identify the incorrect statement.
X and Y are metamers of each other
Both X and Y can show tautomerism
Compound (E) cannot show geometrical isomerism
The number of hyperconjugable H's in (E) is 8

Organic Chemistry
General organic chemistryWrite a balanced equation with the smallest whole number coefficients and include phases, for each
of the following reactions.
a) The combustion of gaseous butane, C4H10, to form gaseous carbon dioxide and water vapor.
b) Aqueous sodium phosphate and aqueous barium bromide react to form aqueous sodium bromide
and solid barium phosphate.
c) Solid iron (II) carbonate reacts with diatomic oxygen gas to form solid iron(III) oxide and carbon
dioxide gas.

Organic Chemistry
General organic chemistryWhich choices are correct explanations about the scanning electron microscope (SEM)? (Select all that apply.)
Useful resolution range for SEM is much narrower than the optical microscopes.
SEM can investigate non conductive samples as good as the conductive ones.
Samples for SEM do not need to be polished nor etched.
Electron beam is the main light source for SEM.
The condensed beam needs to pass through the specimen to obtain the picture for SEM.

Organic Chemistry
General organic chemistryAmmonia will react with fluorine to produce dinitrogen tetrafluoride and hydrogen fluoride as follows:
2NH 3(g) + 5F 2(g) → N ₂F 4( g) + 6HF( g)
How many moles of NH 3 are needed to react completely with 13.6 mol of F ₂?
27.2 mol
34.0 mol
5.44 mol
2.27 mol
6.80 mol

Organic Chemistry
General organic chemistryCalculate the AH of a reaction with bond strengths of bonds broken equal to 60 kcal/mol and 107 kcal/mol and bond strengths of bond formed equal to 110 kcal/mol and 88 kcal/mol.
-245 kcal/mol
151 kcal/mol
31 kcal/mol
-31 kcal/mol

Organic Chemistry
General organic chemistryThe factors that most commonly cause chemical reactions to occur are all the following except
a. formation of a solid
b. formation of a gas
c. formation of water
d. transfer of electrons
e. a decrease in temperature

Organic Chemistry
General organic chemistryWhich of the following is incorrectly named?
Pb(NO3)2, lead(II) nitrate
NH4CIO4, ammonium perchlorate
PO4, phosphate ion
Mg(OH)2, magnesium hydroxide
NO³-, nitrite ion

Organic Chemistry
General organic chemistryIn sickle cell anemia, a mutation occurs and glutamic acid is replaced by valine. What effect would you expect to observe?
Since glutamic acid is a polar and valine is nonpolar, the protein would now change its orientation and move towards the interior in aqueous solution.
Since glutamic acid is a polar and valine is nonpolar, the protein would now change its orientation and move towards the exterior in aqueous solution.
Since glutamic acid and valine are both nonpolar amino acids, both residues would move toward the interior in aqueous solution.
Since glutamic acid and valine are both polar amino acids, both residues would move towards the exterior in aqueous solution.

Organic Chemistry
General organic chemistryAmmonia is produced from the reaction of nitrogen and hydrogen according to the following balanced equation:
N₂(g) + 3H₂(g) → 2 NH3(g)
a What is the maximum mass of ammonia that can be produced from a mixture of 1.20 x 10³ g N₂ and 5.70 x 10² H₂?

Organic Chemistry
General organic chemistryA soda bottle is found to have a length of 0.321 meters. Using unit analysis, show what the length of the soda bottle is in feet.
Use one of the following to set up the conversion factor.
454 g 1 lb 16 oz
1 m39.4 in= 3.28 feet
2.54 cm= 1 in
1 km 0.621 miles
1 L=1.06 qt
3.79 L=1 gal

Organic Chemistry
General organic chemistrya. 1,2,5-trimethylcyclohexane
b. 3-bromo-1-octyne
c. o-iodotoluene
d. pichlorophenol
e. cis-2-isopropylcyclobutanol
f. 2,3-dibromocyclohexene
g. trans-4-methylcycloheptanol
h. 3-methyl-3-pentanol

Organic Chemistry
General organic chemistryAn aqueous magnesium chloride solution is made by dissolving 5.50 moles of MgCl, in sufficient water so that the final volume of the solution is 3.60 L. Calculate the molarity of the MgCl₂ solution.

Organic Chemistry
General organic chemistryWhich of the following is true about small and stable nuclei?
They contain an odd number of neutrons
They contain an equal number of protons and neutrons
They contain an odd number of protons
They contain an even number of protons

Organic Chemistry
General organic chemistryThe structure below is a(n) .....
CH3COCH3
A. ether; dimethyl ether; ether
B. aldehyde; propanal; acetone
C. ketone; propanone; acetone
D. ketone; acetone; propanone
E. ether; propane oxide; acetone
with the IUPAC name......
and the common name

Organic Chemistry
General organic chemistryThe incomplete combustion of propane gas produces among others
A. soot and hydrogen gas
B. soot and carbon monoxide
C. carbon dioxide and hydrogen peroxide
D. aromatic hydrocarbon and sulfur
E. sulfur and nitrogen oxides

Organic Chemistry
General organic chemistryConsider equal mole samples of dinitrogen monoxide, aluminum nitrate, and potassium
cyanide. Rank these from least to greatest number of nitrogen atoms in each sample.
potassium cyanide, dinitrogen monoxide, aluminum nitrate
aluminum nitrate, potassium cyanide, dinitrogen monoxide
dinitrogen monoxide, aluminum nitrate, potassium cyanide
potassium cyanide, aluminum nitrate, dinitrogen monoxide

Organic Chemistry
General organic chemistryAccording to Emerson in
Section 6 of "Nature," in what
way does nature (e.g., going
to the woods) restore our
faith?
A. It causes us to believe that we can
slough off old age and become little
children again.
B. It causes us to believe that nothing can
happen to us in life that "nature" cannot
repair.
C. It causes us to believe that if we just
stay in the woods, everything will be like a
festival.

Organic Chemistry
General organic chemistryIf you were writing that one
of Emerson's purposes in
writing "Self-Reliance" was to
convince his readers to be
themselves, which passage
from the text would you cite
to best support your thesis?
A. Sec 7: I ought to go upright and vital,
and speak the rude truth in all ways.
B. Sec 2: This sculpture in the memory'is
not without preestablished harmony.
C. Sec. 6: The only right is what is after
my constitution; the only wrong what is
against it.

Organic Chemistry
General organic chemistryThe radius of a uranium atom is 149 pm. How many uranium atoms would have to be laid side by side to span a distance of 1.15 mm?

Organic Chemistry
General organic chemistryWhich of these would be considered an example of vertical integration?
Standard Oil controlling 90% of the oil industry
The Industrial Workers of the World (IWW) being a union that was open to all workers, even unskilled immigrants
Political machines providing immigrants with jobs in exchange for votes
Carnegie Steel owning the mines that produced the iron it would make into steel, as well as owning the boats that brought the iron to the steel mills

Organic Chemistry
General organic chemistryFor the following reaction, 6.09 grams of perchloric acid (HCIO4) are mixed with excess tetraphosphorus decaoxide.
The reaction yields 1.86 grams of phosphoric acid.
perchloric acid (HClO4) (aq) + tetraphosphorus decaoxide (s)-phosphoric acid (aq) + dichlorine heptaoxide (1)
What is the theoretical yield of phosphoric acid ?
What is the percent yield of phosphoric acid ?

Organic Chemistry
General organic chemistry2C6H5 Cl + C₂ HOCl3 → C14H9 Cl5 + H₂O
In a government lab, 1167 g of chlorobenzene is reacted with 486 g of chloral.
a. What mass of DDT is formed, assuming 100% yield?
b. Which reactant is limiting? Which is in excess?
Mass
.
c. What mass of the excess reactant is left over?
d. If the actual yield of DDT is 288.0 g, what is the percent yield?
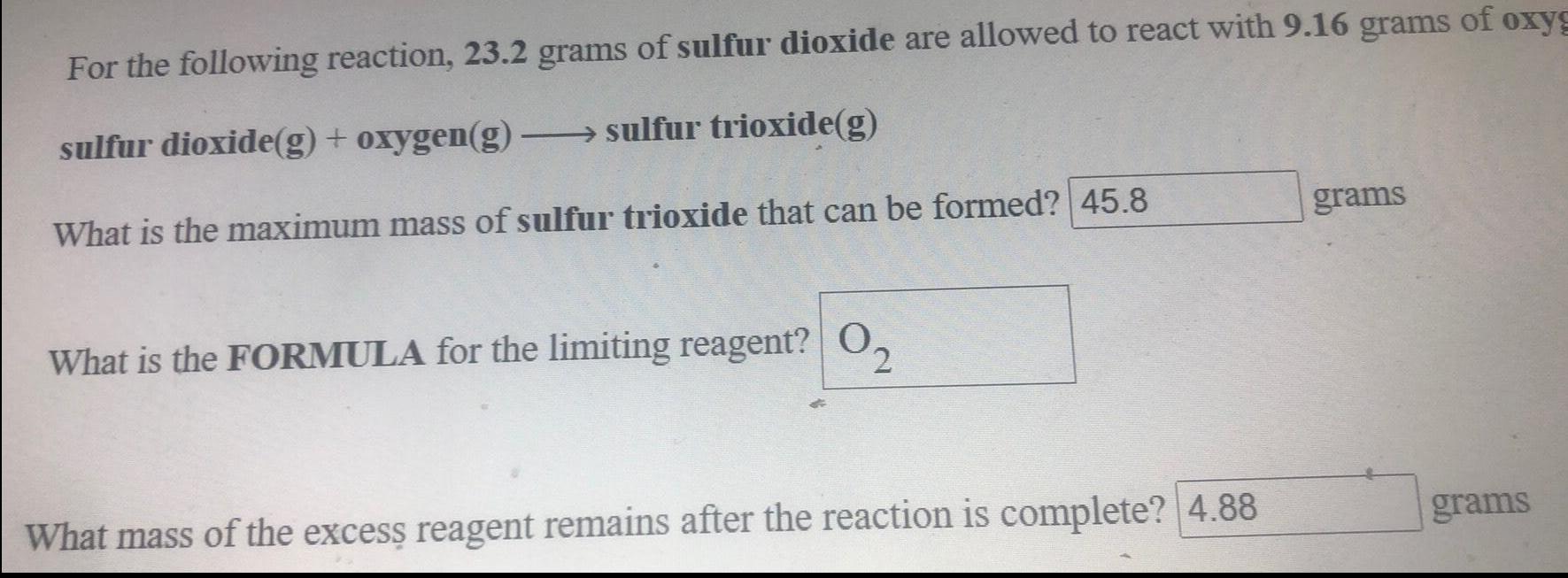
Organic Chemistry
General organic chemistryFor the following reaction, 23.2 grams of sulfur dioxide are allowed to react with 9.16 grams of oxygen
sulfur dioxide(g) + oxygen(g) →→→sulfur trioxide(g)
What is the maximum mass of sulfur trioxide that can be formed?
What is the FORMULA for the limiting reagent?
What mass of the excess reagent remains after the reaction is complete?

Organic Chemistry
General organic chemistryFor the following reaction, 9.93 grams of nitrogen monoxide are allowed to react with 8.91 grams of oxygen gas.
nitrogen monoxide(g) + oxygen(g) → nitrogen dioxide(g)
What is the maximum mass of nitrogen dioxide that can be formed?
What is the FORMULA for the limiting reagent?
What mass of the excess reagent remains after the reaction is complete?

Organic Chemistry
General organic chemistryDraw the expanded structural formula for 1,3-dichlorocyclopentane. An expanded structural formula shows all the atoms of the molecule and all the
bonds between the atoms in the molecule.

Organic Chemistry
General organic chemistryConsider the common arrow types used in organic chemistry listed below. Ordered list
A. nucleophilic attack by a single atom
B. heterolytic o bond cleavage
C. nucleophilic attack by a w bond
D. bond dissociation
E. bond formation
Which of these types are the reverse of each other?

Organic Chemistry
General organic chemistryWhat is the name of the compound with the formula CO₂?
What is the name of the compound with the formula NF3 ?
What is the name of the compound with the formula N₂O ?

Organic Chemistry
General organic chemistryWhich of the following statements is valid?
2-butene; enantiomers
2,4-dimethylhexane; superimposable mirror images
2-bromobutane;
achiral molecule
propene; center of asymmetry.

Organic Chemistry
General organic chemistryClassify the following as a heterogeneous mixture, homogeneous mixture (solution), or a pure substance: hot coffee with sugar (completely dissolved)
A. heterogeneous mixture
B. homogeneous mixture/solution
C. pure substance

Organic Chemistry
General organic chemistryThe equation below represents the acid catalyzed ____ of ____ to form
USE IUPAC NAMES ONLY
CH2=CH-CH3 + H₂=====> CH3CH(OH)CH3
A. carboxylation; propyne; 1-propanol
B. reduction; 2-propene; 1-propanol
C. hydration; propene; 2-propanol
D. hydration; propane; dimathylether
E. hydrogenation; propene; 2-propanol

Organic Chemistry
General organic chemistryEthanol, CH3CH₂OH, has a pKa value of 15.9 while acetic acid, CH3COOH, has a pKa value of 4.74. This data indicates that
ethanol is a stronger acid than acetic acid.
the conjugate base of ethanol is a stronger base than that of acetic acid.
the conjugate base of ethanol would not react with acetic acid.
the ratio of acetic acid to its conjugate base is larger than that of ethanol

Organic Chemistry
General organic chemistryWhich of the following objects contains at least one plane of symmetry?
Hint 1. How to determine planes of symmetry
wooden chair
work glove
a pair of scissors
metal screw

Organic Chemistry
General organic chemistryYou were running GC experiments on your unknown as well as unknown + sample spikes. Your unknown had two components, but when you got to the last sample containing a "spike,"your syringe became clogged. Since you were in a hurry, you quickly borrowed a syringe from the neighboring instrument; however, you ended up with four peaks in your chromatogram! What is the easiest and fastest thing to do to finish your lab?
Re-mix all of your samples and run all the chromatograms again.
Re-run your last sample after rinsing the syringe with that sample.
Run the sample on a different GC.
Answers A and B
Answers A and C
Answers B and C

Organic Chemistry
General organic chemistryWhich of the following is NOT an important consideration in planning a recrystallization of compound X in solvent Y:
the solubility of X in solvent Y at the boiling point of Y
the boiling point of Y
the solubility of X in solvent Y at 0 °C h
the molecular weight of X
the solubility of known impurities in solvent Y

Organic Chemistry
General organic chemistryWe saw in lab that although iodine is a much bigger atom than chlorine, iodine has a much lower conformational energy cost than
chlorine as an axial substituent.
What best explains this phenomenon?
The very long carbon-iodine bond minimizes steric strain.
The very long carbon-iodine bond minimizes torsional strain.
The outermost electrons of iodine are much more dispersed and therefore strain energy is reduced.
Olodine's heavier atomic weight allows it to reside further away from the chair structure.

Organic Chemistry
General organic chemistryIn this lab you synthesized Methyl acetoacetate via a Claisen Condensation reaction, which is combining two carbonyl containing molecules in the presence of a base. What functional group(s) were present in these two carbonyl reactants needed for this reaction?
Two esters
Two aldehydes
An ester and a ketone
Two ketones

Organic Chemistry
General organic chemistryA gaseous system that is 160.0 K and with a pressure of 126.6 kPa, has its temperature changed to 267.6 K. What is the new pressure of the system? Do not include units in your answer, they are presumed to be kPa. Round your answer to the nearest hundredth.

Organic Chemistry
General organic chemistryWhich of the following helps us to predict the effect of change in temperature on a reaction system?
Select the correct answer below:
Collision theory
The ideal gas law
Le Châtelier's principle
The effects of changes in temperature on equilibrium reactions are entirely random and cannot be predicted.

Organic Chemistry
General organic chemistryWhich statement about solutions is true?
Select the correct answer below:
The solute must be the same physical state as the solvent.
A solution can have only one solute.
A solution can have many solutes.
Given enough time, the solute will separate from the solvent.

Organic Chemistry
General organic chemistryConsider the reaction below. According to the Bronsted-Lowry definition, what acts as
the conjugate acid when the reaction proceeds in the forward direction?
CN + H₂O = OH + HCN
Select the correct answer below:
CN
OOH
OHCN
H₂O

Organic Chemistry
General organic chemistryA solution of a compound in water conducts electricity, turns litmus red, and has a sour taste. What compound might be in the solution?
Select the correct answer below:
NaHCO3
Mg(OH)2
C3H5(COOH)3
NH3

Organic Chemistry
General organic chemistryWhich of the following phenomona is characteristic of unstable nuclei as opposed to stable nuclei?
Select the correct answer below:
Radioactivity
Multiple isotopes
Chemical reactivity
Atomic masses (in amu) unequal to the mass number of the isotope.

Organic Chemistry
General organic chemistryWhich of the following two compounds is a stronger base: Sodium hydrosulfide (NaSH) or sodium hydroxide (NaOH)? In 1-2 complete and concise sentences, explain your logic in making this assignment of base strength.

Organic Chemistry
General organic chemistryAn ester of formula C7H1402 gives an alcohol and an acid when hydrolyzed. When the alcohol is
isolated and oxidized, it forms a ketone. Which of these compounds could be the ester?
b. sec-butyl propanoate
d. tert-butyl propanoate
a. isopropyl propanoate
c. ethyl 2,2-dimethylpropanoate

Organic Chemistry
General organic chemistryFor a certain reaction K is known to be 0.003. The reaction quotient was found to be
0.003
Which way will the reaction proceed?
towards the products
not enough info to know
it is at equilbrium
towards the reactants

Organic Chemistry
General organic chemistryWhat is the average rate of production of carbon dioxide for the system between 2.0 and 4.0 minutes, CH4(g)+2O2(g)->CO2(g)+2H₂O(g), if the concentration of carbon dioxide is 2.5 mol/L after 2.0 minutes and 7.2 mol/L after 4.0 minutes?
2.35 mol/(L'min)
1.18 mol/(L*min)
42.6 mol/(L*min)
0.426 mol/(L* min)
0.952 mol/(L' min)