Practical Detection Questions and Answers

Organic Chemistry
Practical DetectionThese words are spoken by Hester to Dimmesdale when the townspeople wanted to separate
Hester and her child.
"Speak thou for me!' cried she. Thou wast my pastor, and hadst charge of my soul, and knowest me
better than these men can. I will not lose the child! Speak to me! Thou knowest, for thou hast
sympathies which these men lack! - thou knowest what is in my heart, and what are a mother's
rights, and how much the stronger they are when that mother has but her child and the scarlet
letter! Look thou to it! I will not lose the child! Look to it!"
Which among the following lines/phrase thát Hester speaks to convey a subtle message to
Dimmesdale?
"Speak thou for meľ' cried she."
"Thou knowest, - for thou hast sympathies which these men lack!"
'Thou knowest what is in my heart"
"How much the stronger they are, when that mother has but her child and the scarlet letter!"

Organic Chemistry
Practical DetectionPoints 2
The dominant intellectual movement of the Renaissance was called
Romanticism
Feudalism
Humanism
Classicism
Organic Chemistry
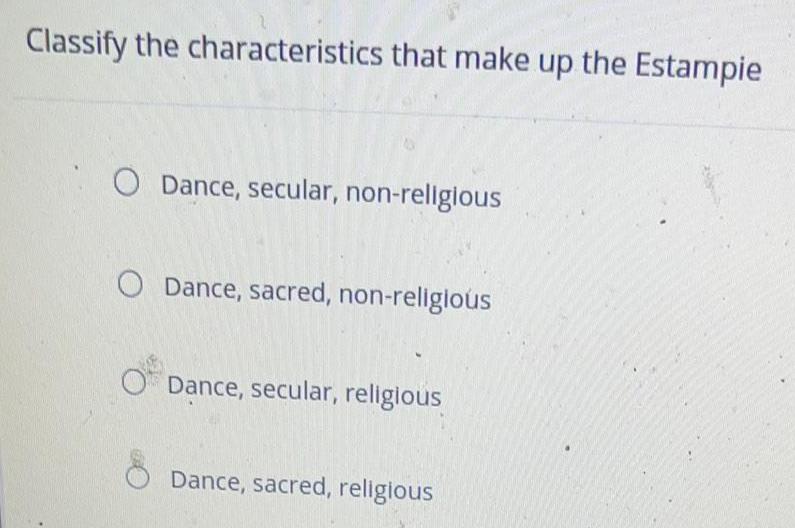
Practical DetectionClassify the characteristics that make up the Estampie
Dance, secular, non-religious
Dance, sacred, non-religious
Dance, secular, religious
Dance, sacred, religious

Organic Chemistry
Practical DetectionAir is a mixture of nitrogen, oxygen, and argon
(and a few other trace gases that we will ignore
here). Assume you have a container of air,
where nitrogen has a partial pressure of 761
torr, oxygen has a partial pressure of 226 torr,
and argon has a partial pressure of 37 torr.
What is the total pressure in the container?
Your Answer:
![A sample of calcium carbonate [CaCO3 (s)] absorbs 40.3 J of heat, upon which the temperature of
the sample increases from 20.8 °C to 27.3 °C. If the specific heat of calcium carbonate is 0.82 J/g-
K, what is the mass (in grams) of the sample?
-7.6
7.6
5.1
5.3
0.13](https://media.kunduz.com/media/sug-question/raw/52516953-1658947478.274892.jpeg?w=256)
Organic Chemistry
Practical DetectionA sample of calcium carbonate [CaCO3 (s)] absorbs 40.3 J of heat, upon which the temperature of
the sample increases from 20.8 °C to 27.3 °C. If the specific heat of calcium carbonate is 0.82 J/g-
K, what is the mass (in grams) of the sample?
-7.6
7.6
5.1
5.3
0.13

Organic Chemistry
Practical DetectionCalculate the work done in kJ during a reaction in which the internal volume contracts from 48 L to
19 L against an outside pressure of 2.5 atm. (1 L-atm = 101.3 J)
-7300
7.3
7300
0, no work is done.
-7.3

Organic Chemistry
Practical DetectionNicholas reveals his aunt's
manipulation when he brings u..
the strawberry jam incident and
concludes, "Oh, Devil, you have
sold yourself!" (Saki 5).
A. Passage #1
B. Passage #2
C. Passage #3
D. Passage #4

Organic Chemistry
Practical DetectionIn the chunked method, you ca 6
sometimes put a bit of evidence
into your explanation. Which is an
example of explanation that
contains a bit of evidence?
A. The only winner, it seems, are the wolves,
who work in unison...
B. The men fight over a land "where the trees
can't even stand upright in a breath of wind"
(Saki 5)...
C. In this story, everything is ironic.

Organic Chemistry
Practical DetectionFrom the topic sentence in the 5
sample paragraph, Saki's use or
which literary device or technique
is being addressed?
A. characterization
B. irony
C. dialogue

Organic Chemistry
Practical DetectionUsing the alternating method, 7
where does the following
explanation belong in the outline
of Body Paragraph 2?
The wolves symbolize both the
power of nature and its disregard
for men or their concerns.
A. Explanation 1
B. Explanation 2
C. Explanation 3

Organic Chemistry
Practical DetectionFrom the sample conclusion
paragraph, which passage is a
Paraphrase of the text/story?
A. In "The Lady, or the Tiger" Stockton
B. The story is about a princess who must
choose whether she would prefer to see her
lover die...
C. It is clear that Stockton uses symbolism
throughout the story; the tiger represents
jealousy...
D. Stockton...thematically suggests that love
never ends well no matter what the
circumstances

Organic Chemistry
Practical DetectionAccording to the following balanced chemical reaction:
2 Cr(s) + 2 H3PO4 (aq) + 3 H₂ (g) + 2 CrPO4
-
What mass (in grams) of H₂ Las is produced when 48.3 grams of phosphoric acid
are reacted with excess chromium?

Organic Chemistry
Practical DetectionWhich statement is true about how scientists draw conclusions from data?
A. Scientists do not allow others to make conclusions about their
data.
B. Two scientists may have different underlying assumptions that
lead them to different conclusions about the same data.
C. Any two scientists will always come to the same conclusion about
a data set.
D. Teams of scientists never share their data to help other teams
draw conclusions.

Organic Chemistry
Practical DetectionHow does the phase of water affect its specific heat capacity?
A. Water vapor has the highest specific heat capacity.
B. All phases have the same specific heat capacity.
C. Frozen water has the highest specific heat capacity.
D. Liquid water has the highest specific heat capacity.

Organic Chemistry
Practical DetectionCalculate the number of carbon atoms in a 140.0 g sample of camphor (C₁0H160).
Be sure your answer has a unit symbol if necessary, and round it to 4 significant digits.

Organic Chemistry
Practical DetectionAccording to the following balanced chemical reaction:
CH4(g) + 2 O₂(g) - CO₂(g) + 2 H₂O(l)
4
What mass (in grams) of CO₂ is produced when 56.5 grams of oxygen are reacted with excess CH4?
Your Answer:
Answer
units

Organic Chemistry
Practical DetectionThe density of iron is 7.874.Convert to kilogram per cubic meter.
Do not include units in your answer.
Provide your answer below:

Organic Chemistry
Practical DetectionPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains
state symbols after every reactant and product.
HCIO3(aq) + H2o ⇒ _

Organic Chemistry
Practical DetectionIdentify areason that chemical reactions release energy during the reaction process.
Select one:
a. forming bonds
b. breaking bonds
c. storing energy
d. overcoming activation energy

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Running at a rate of 7 mi/h uses 812 Calories in an hour. Convert this value to (a) J/h and (b) kJ/h.
Report answers to appropriate number of significant figures.

Organic Chemistry
Practical DetectionWhat is the net ionic equation of the reaction of ZnCl2 with NaOH?
Express you answer as a chemical equation including phases.
Organic Chemistry
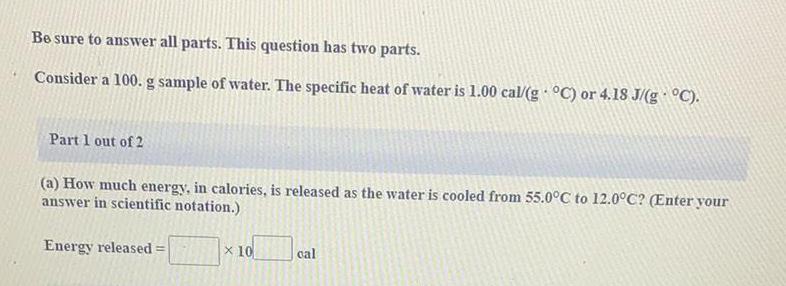
Practical DetectionBe sure to answer all parts. This question has two parts.
Consider a 100. g sample of water. The specific heat of water is 1.00 cal/g °C) or 4.18 J/(g · °C).
Part 1 out of 2
(a) How much energy, in calories, is released as the water is cooled from 55.0°C to 12.0°C? (Enter your
answer in scientific notation.)
Energy released = _ x 10 _ cal

Organic Chemistry
Practical DetectionAccording to the definitions
given, which is the correct
use of the word novel as an
adjective?
A. Wyatt came up with the novel idea of
having a talk show just for students.
B. This book about Abraham Lincoln is a
very interesting novel.
C. Something that everyone already
knows or is doing is called novel.

Organic Chemistry
Practical DetectionQuestions (Answer in complete, full sentences. Limit yourself to the available space.)
1. Using your knowledge of this reaction, sketch an energy profile diagram for the reaction. In
your diagram, clearly label the axes, structures of reactants, structures of products,
activation energy (E₂), transition state structure, and AH.

Organic Chemistry
Practical DetectionFor scuba dives below 150 ft, helium is often used to replace nitrogen in the scuba tank. If 14.7 g of He(g) and 28.0 g of O₂(g) are added to a previously evacuated
5.35 L tank at 20.°C, calculate the partial pressure of each gas present as well as the total pressure in the tank.
Partial pressure of He
Partial pressure of 02
Total pressure =
atm
atm
atm

Organic Chemistry
Practical DetectionWhy does Hess's law allow you to determine the enthalpy change of a
reaction?
A. It states how the equilibrium constant is related to the enthalpy
change.
B. It states that the change in enthalpy depends on the reaction
pathway between the products and the reactants.
C. It states that the change in enthalpy is independent of the pathway
the reaction takes to get to the products.
D. It states that the enthalpy of any reaction can be determined using
a bomb calorimeter.

Organic Chemistry
Practical DetectionA box has volume of 4.75 x 1018 μm³ (cubic micrometers). What is the volume of the box in cubic feet (ft³)?
2.54 cm = 1.00 inch; 12.00 inches = 1.00 foot
1.68 x 1011 ft³ 1.55 x 1013 ft³
2.87 × 1017 ft³ O
1.87 × 106 ft³
168 ft³

Organic Chemistry
Practical DetectionA sample of neon gas at 302 K and 0.565 atm occupies a volume of 4.91 L. If the pressure of the gas is decreased, while at the same time
it is heated to a higher temperature, the final gas volume
will be larger than 4.91 L.
could be larger or smaller than 4.91 L depending on the final pressure and temperature.
will be smaller than 4.91 L.

Organic Chemistry
Practical DetectionYou would like to make a solution that 38.8 L of a 2.9 M solution. You have a stock solution that is 9 M. How much your stock solution will you need
to dilute to get the solution you want?
Just the number!

Organic Chemistry
Practical DetectionAccording to Le Châtelier's principle, how would a change in pressure affect a
gaseous system in equilibrium?
A. The equilibrium would shift to reduce the pressure change.
B. The number of both reactants and products would increase.
C. The equilibrium would shift in the direction of the change.
D. All equilibrium interactions would stop.

Organic Chemistry
Practical DetectionYou work at a factory that produces
ammonia gas. The reaction gives off
heat. If the reaction is at equilibrium,
how can you force the reaction to
produce more products?
Please choose the correct answer.
Increase the temperature.
Decrease the temperature.
Changing the temperature has
no effect.

Organic Chemistry
Practical DetectionWhich of the statements below are true?
A. Round bottom flasks always have 14/20 glass joints
B. Round bottom flasks usually are equipped with male joints
C. When greasing glassware, you should always grease the joint of the round bottom flask
D. Round bottom flasks are only used to heat the reaction mixtures
E. Round bottom flasks are usually equipped with female joints
F. Round bottom flasks should be filled just to 1/3 of the volume.

Organic Chemistry
Practical DetectionBarium can be analyzed by precipitating it as BaSO4 and determining the mass of the precipitate. When a 0.269-g sample of a
barium compound was treated with excess H₂SO4, 0.0891 g of BaSO4 formed. What percentage of barium is in the compound?

Organic Chemistry
Practical Detection4 The black "tarnish" that can develop on silver objects is a result of the metal reacting with sulfur-containing compounds in the
air to produce silver sulfide. A sample of silver sulfide was found to contain 90.64 g of silver and 13.47 g of sulfur. Use these data
to calculate the empirical formula of silver sulfide.

Organic Chemistry
Practical DetectionYou analyzed 2.475 g of a mixture of NaCl, SiO₂, and CaCO. You isolate 0.825 g NaCl, 0.825 g of CaCO3, and 0.580 g SiO₂. You were told that the original mixture contained 35% NaCl, 45% CaCO3, and 20% SiO₂. Report two possible sources of error.

Organic Chemistry
Practical DetectionYou time travel 100 years into the future and learn that several new elements have
been discovered, as pictured. These elements are frequently found as oxides, and
need to be separated in order to extract the pure element. Before going through
this effort, it is useful to know what amount of the element can be extracted. What
is the mass percent composition of element A in the compound A505?

Organic Chemistry
Practical DetectionDr. A needs to make 125.0 mL of a 0.225 M solution of BaCl2. What mass (in grams)
of barium chloride is needed to prepare this solution?
Report your answer to the correct number of significant figures. You may show your
work here or on a separate sheet of paper to receive partial credit.

Organic Chemistry
Practical DetectionCalculate the molality of a solution that contains 78.2 grams of Solute in 278.8 mL
of solution.
The following information may be useful for this calculation.
The molar mass of Solute = 120.71 g/mol
The density of the solution = 1.0 g/mL

Organic Chemistry
Practical DetectionA chemist must dilute 55.5 mL of 23.0 µM aqueous magnesium fluoride (MgF₂) solution until the concentration falls to 17.0 μM. He'll do this by adding
distilled water to the solution until it reaches a certain final volume.
Calculate this final volume, in milliliters. Be sure your answer has the correct number of significant digits.

Organic Chemistry
Practical Detection7) The percent composition of an unknown substance is 75.42 % Carbon, 6.63 %
Hydrogen,8.38 % Nitrogen, and 9.57 % Oxygen. If its molar mass is 334.0 g/mol
what is its empirical and molecular formula?

Organic Chemistry
Practical DetectionA hospital buys a compound that contains an isotope of barium. Several months later, most of the barium has changed to the
element lanthanum. Which of the following is responsible for this change?
reactions with the container wall
exposure to air
radioactive decay
absorption of moisture from the air

Organic Chemistry
Practical DetectionA patient at risk for cardiovascular disease is found to have a blood LDL concentration of 180 mg/dL.
Calculate the total mass of LDL circulating in the blood of this patient given the total volume of blood is 5.3 L.
0.94 g
2.9 g
34 g
9.5 g

Organic Chemistry
Practical DetectionHopkinsium (Hk) is an element that has not been discovered yet. There are 210
grams in one mole of Hk. If a chemist reacts 4.6 grams of Hk according to the
following reaction, how many grams of HkBr will form? 2 Hk + Br₂ → 2 HkBr *
-

Organic Chemistry
Practical DetectionCalculate the number of molecules of oxygen present in 0.125 moles of O₂. (1 mole =
6.022 1023 objects). Show all your working clearly.
A) 3.01 x 1024 molecules
B) 7.53 x 1022 molecules
C) 0.125 molecules
D) 6.022 x 1023 molecules

Organic Chemistry
Practical DetectionThe boiling of water is a:
chemical change because heat is needed for the process to occur.
physical change because the gaseous water is chemically the same as the liqui
physical change because the water merely disappears.
chemical change because a gas (steam) is given off.
chemical and physical damage.

Organic Chemistry
Practical DetectionWhich of the following has a different value on the moon compared to earth?
Moles
Mass
Weight
Time
![Identify the highlighted element in the periodic table and give its [1] element name and symbol;
[2] group number; [3] period; [4] classification (i.e., main group element, transition metal, or inner
transition metal).
[1] Element name (symbol)
[2] Group number (select)
[3] Period
[4] Classification (select)](https://media.kunduz.com/media/sug-question/raw/49546562-1658863994.6474319.jpeg?w=256)
Organic Chemistry
Practical DetectionIdentify the highlighted element in the periodic table and give its [1] element name and symbol;
[2] group number; [3] period; [4] classification (i.e., main group element, transition metal, or inner
transition metal).
[1] Element name (symbol)
[2] Group number (select)
[3] Period
[4] Classification (select)

Organic Chemistry
Practical DetectionHow old is a piece of cotton cloth if the half-life of carbon-14 is 5,730 years, and the
carbon-14 composition of the cloth is 22 percent that of living plants?
A) 1,813 years
B) 12,517 years
C) 1.21 x 10-4 years ¹
D) 1.21 x 10-4 years

Organic Chemistry
Practical DetectionIn the course of their desolate wanderings Oedipus and Antigone came to Colonus, a lovely spot near Athens, where the one-time Erinyes, the Furies, now the Benignant Goddesses, had a place sacred to them and therefore a refuge for suppliants. The blind old man and his daughter felt safe there, and there Oedipus died. Most unhappy in much of his life, he was happy at the end. The oracle which once had spoken terrible words to him comforted him when he was dying. Apollo promised that he, the disgraced, the homeless wanderer, would bring to the place where his grave should be a mysterious blessing from the gods. Theseus, the King of Athens, received him with all honor, and the old man died rejoicing that he was no longer hateful to men, but welcomed as a benefactor to the land that harbored him.
Which statement accurately analyzes this passage from a historical perspective?
(A) People who heard this story when it was first told may have believed that oracles could predict the future accurately
(B) The characters in this story live in a society where everyone is equal.
(C) The characters in this story are kind to one another, even when being kind turns out to be risky or frightening
(D) People who heard this story when it was first told did not believe in sacred spaces.

Organic Chemistry
Practical DetectionHow many joules of energy must be added to completely convert 24.0 g of ice at 0 °C to liquid water at 0 °C? The heat of fusion of water is 334 J/g, and the heat of vaporiztion of water is 2257 J/g.