Practical Detection Questions and Answers

Organic Chemistry
Practical DetectionHow many molecules of carbon dioxide will be formed if 6.71 g of propane is burned in the following reaction?
C₂H₂(g) +50₂(g) →3CO₂(g) + 4H₂O(g)
1.21 x 1025 molecules
9.16 x 1022 molecules
3.67 x 1023 molecules
2.75 x 1023 molecules
4.58 x 1023 molecules

Organic Chemistry
Practical DetectionIf 4.00 L of water vapor at 50.2 °C and 0.121 atm reacts with excess iron, how many grams of iron(III) oxide will be produced?
2 Fe(s) + 3 H₂O(g)-->Fe₂O₂ (s) + 3 H₂(g)

Organic Chemistry
Practical DetectionIf standard heat of formation of CO₂ is - 400 kJ/mol. The amount of C(graphite) required, if 10 kJ heat is released during the combustion, is g.

Organic Chemistry
Practical DetectionTwo elements,
R and
Q, combine to form two binary compounds. In the first
compound, 28.0 g of
R combines with 3.00 g of
Q. In the second compound, 7.00 g of
R combines with 4.50 g of
Q. Show that these data are in accord with the law of
multiple proportions. If the formula of the second
compound is
RQ, what is the formula of the first compound?
Compound I:||

Organic Chemistry
Practical DetectionA chemist makes 490. mL of potassium permanganate (KMnO4) working solution by adding distilled water to 110. mL of a 0.391 mol/L stock solution of
potassium permanganate in water. Calculate the concentration of the chemist's working solution. Round your answer to 3 significant digits.

Organic Chemistry
Practical DetectionWhat is the mass number for an atom of molybdenum containing 42 protons and 53 neutrons?

Organic Chemistry
Practical DetectionIf you added Na2CO3 to Hcl bubbles would be formed on adding the two reeactants. If you lit a match in the neck of the test tube the flame would immediately be extinguished. Based on the above observations, answer the following questions:
a. What evidence is there of a chemical reaction?
b. What caused the change in the burning match or split?
c. Write a balanced equation for this reaction.
d. What type of chemical reaction is this?

Organic Chemistry
Practical DetectionWhich of the following correctly summarizes the Pauli Exclusionary Principle?
Each electron within an atom will have its own unique set of four quantum numbers.
Electrons orbiting an atom fill the lowest available energy levels prior to filling higher energy orbitals.
Matter can neither be created nor destroyed. It only changes form during chemical reactions. The ground state or lowest energy state of an atom is the one that contains the maximum number of unpaired electrons.
It is fundamentally impossible to know both the location and velocity of an electron at the same time.

Organic Chemistry
Practical DetectionSolid manganese (IV) oxide reacts with solid aluminum metal to produce solid manganese and solid aluminum oxide. Balance the equation for this reaction (in lowest multiple integers).

Organic Chemistry
Practical DetectionAn experiment is set up to determine the type of radioactive decay coming from a certain hazardous nuclide. The analytical instrument used in this experiment detects the charge of the particles emitted from the nuclide. When interpreting the results of the experiment, it was discovered that the emitted particles had no charge at all. Which of the following could be the identity of these particles?
Select the correct answer below:
Positrons
Protons
y particles
β particles

Organic Chemistry
Practical DetectionSeveral steps are involved in the industrial production of sulfuric acid. One step involves the oxidation of sulfur dioxide gas to form sulfur trioxide gas. A catalyst is used to increase the rate of production of sulfur trioxide gas. In a rigid cylinder with a movable piston, this reaction reaches equilibrium, as represented by the equation below.
2SO₂(g) + O₂(g) →2SO3(g) + 392 kJ
A. Explain in terms of collision theory, why increasing the pressure of the gases in the cylinder increases the rate of the forward reaction.
B. State, in terms of the concentration of SO3(g), what occurs when the following changes are made to the equilibrium mixtures.
i. More O₂(g) is added.
ii. SO3(g) is removed.
C. If 40 grams of sulfur trioxide gas, SO3(g) was passed through 250 mL of water, determine the olarity of sulfuric acid formed.

Organic Chemistry
Practical DetectionAssume you are trying to extract pure iron (Fe) from a sample of Fe2O3. What mass (in grams) of iron can be extracted from 75.0 grams of Fe2O3?

Organic Chemistry
Practical DetectionIron (III) oxide reacts with carbon monoxide according to the equation:
Fe2O3(s) + 3 CO(g) → 2 Fe(s) + 3 CO2(g)
a) If 22.55 g Fe2O3 is reacted with excess CO, how many grams of iron can be made?
b) If we want to refine 10.0 kg of iron metal, how many moles of carbon monoxide gas do we need?
c) If 2.50 moles of Fe2O3 is reacted with carbon monoxide, how many liters of carbon dioxide gas form?

Organic Chemistry
Practical Detection4. How many grams of acetylene would be given off if the limiting reagent in the reaction is calcium
carbide and you start with 2.00 g?
molar mass CaC₂ = 64.1 g/mol molar mass C₂H₂ = 26.0 g/mol
Show work. Make sure your answer is clearly indicated, and circle your answer if you upload an image
of your work.

Organic Chemistry
Practical DetectionGive the name for each of the following compounds, which contain a polyatomic ions
a. CuHPO4:
b. LiClO₂:
c. Pb(SO4)2:

Organic Chemistry
Practical DetectionWhich statements below that is true of ß decay?
Select the correct answer below:
OIt is most likely to occur when the nucleus is in an excited state.
It decreases the nuclide's mass number.
It increases the nuclide's mass number.
It is most likely to occur when the neutron:proton (n:p) ratio of the nuclide is too large.

Organic Chemistry
Practical DetectionUnlike a change in concentration or pressure, a change in temperature will affect the
Select the correct answer below:
rate of the forward reaction
rate of the reverse reaction
equilbrium constant
none of the above

Organic Chemistry
Practical DetectionAccording to VSEPR theory, molecules adjust their shapes to keep which of the
following as far apart as possible?
pairs of valence electrons
inner shell electrons
bonding pairs of electrons
mobile electrons
lone pairs of electrons

Organic Chemistry
Practical DetectionWhich of the following is the electron configuration for aluminum in the ground
state? (The numbers after the letters are meant to be superscript)
1s2 2s2 2p5 3s1 3p1
1s2 2s2 2p6 3s2 3p1
1s2 2s2 2p6 3s1 3p2
1s2 2s2 2p6 3s1 3p1
1s2 2s2 2p6

Organic Chemistry
Practical Detection12. Marketing people typically have the following basic objectives to accomplish. Which one below is
not one of them:
a. Focus only on developing new markets.
b. Maximize the sales of existing products in existing markets.
c. Develop and sell new products.
d. Provide the quality of service necessary for customers to be satisfied with their transactions and to
continue doing business with the organization.

Organic Chemistry
Practical Detection6. The following characteristic applies to which of the eight characteristics of success of sales: They
can match up their product's benefits with the customer's needs.
a. Use the Golden Rule of Selling
b. Sales knowledge at M.D. level
c. Excels at Strategic thinking
d. Stamina for the challenge

Organic Chemistry
Practical DetectionWhat did classical physics predict about the structure of the atom?
Select the correct answer below:
Based on electrostatic attraction, the electrons are repelled by the protons in
the nucleus.
Based on electrostatic attraction, the electrons would spiral into the nucleus.
Based on electrostatic attraction, the electrons were attracted to other
electrons.
Based on electrostatic attraction, atoms are inherently stable.

Organic Chemistry
Practical Detection3. Identify the limiting reactant and the theoretical yield of phosphorous acid if 3.00 mol
of PCl3 is mixed with 3.00 mol of water.
vnnnnn
PC13 + H₂O
H3PO, +
HCI
4. In number 3, how much of the excess reactant is left over?
What is the percent yield if only 58.7 g of H3PO3 is created?

Organic Chemistry
Practical DetectionA drink sold in a health food store contains 2.50% (w/v) of vitamin C. What volume would you have to
ingest to obtain 1,000. mg of vitamin C?
mL solution

Organic Chemistry
Practical DetectionEnter your answer in the provided box.
If a solution contains 240. mEq/L of Na*, how many milliequivalents of Nat are present in 440. mL of
solution?

Organic Chemistry
Practical DetectionHow many molecules of O2 are required to react with 3.6 mol H₂?
2 H₂ + O₂ → 2H₂O
• Use 6.022 x 1023 mol-¹ for Avogadro's number.

Organic Chemistry
Practical DetectionAccording to the below equation, how many moles of P2H4 will be required to
generate 1.76 x 1023 molecules of P4?
6P2H48PH3 + P4
• Use 6.022 x 1023 mol-¹ for Avogadro's number.
• Your answer should have three significant figures.
Provide your answer below:
mol

Organic Chemistry
Practical DetectionBased on the following equation, if we want to produce 25 g NaCl, how many grams
of MgCl₂ would we need?
MgCl, + 2NaOH → MgOH, + 2NaCl
Select the correct answer below:
13 g MgCl₂
20. g MgCl₂
26 g MgCl₂
50. g MgCl₂

Organic Chemistry
Practical DetectionClick in the answer box to activate the palette.
Predict the precipitate produced by mixing an Al(NO3)3 solution with a NaOH solution. Write the net
ionic equation for the reaction. (Include the states of matter.)

Organic Chemistry
Practical DetectionWhich pairs of elements are likely to form ionic bonds and which pairs are likely to form covalent
bonds?
a. lithium and fluorine
(select)
c. two nitrogen atoms
(select)
b. selenium and oxygen
(select)
d. carbon and oxygen
(select)

Organic Chemistry
Practical Detection5. Write the formula for the following compounds.
A) Lithium acetate
B) Magnesium hydroxide
C) Mercury (I) chloride
D) Manganese (III) fluoride
E) Ammonium sulfate
F) Silver sulfide
G) Nickel (II) nitrate
H) Gold (I) nitride
I) Barium phosphide
J) Calcium cyanide_
K) Lead (IV) sulfite
L) Chromium (III) chlorate
M) Copper (II) hypochlorite
N) Trinitrogen pentasulfide
O) Dihydrogen dioxide
P) Iron (III) nitrite
Q) Carbon tetrachloride
R) Sulfur hexafluoride

Organic Chemistry
Practical DetectionAcids and bases can neutralize each other in double displacement reactions. For
example, if hydrogen chloride (a strong acid, HCl) is mixed with sodium hydroxide (a
strong base, NaOH), the products are water, H₂O, and salt, NaCl. Write the
balanced double displacement reaction with the simplest whole number coefficients.

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Using the balanced equation for the combustion of acetylene, answer the following questions.
2H_C=C_H+502→4CO2+2 H,O
a. How many moles of O₂ are needed to react completely with 2.60 mol of C₂H₂?
mol O₂
b. How many moles of C₂H₂ are needed to form 0.66 mol of CO₂?
mol C₂H₂

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Some coal is high in sulfur (S) content, and when it burns, it forms sulfuric acid (H₂SO4), a major
component of acid rain, by a series of reactions. Balance the equation for the overall conversion draw
below.
S(s) + O₂(g) + H₂O(1)→ H₂SO4(aq)

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Give the electronic configuration for antimony and then convert it to noble gas notation. (Give the
electronic configuration first and the noble gas notation second with the orbitals in order of increasing
energy.)
antimony:
9

Organic Chemistry
Practical DetectionAn unknown amount of gas occupies 32.5 L at 4.71 atm and 298 K. How many moles does the sample
contain? What is the mass if the gas is helium? What is the mass if the gas is argon?
mol
If the gas is helium:
If the gas is argon:
g

Organic Chemistry
Practical DetectionBe sure to answer all parts.
Calculate the volume of each substance at STP.
a. 4.8 mol Ar
L
b. 1.3 g CO₂
L

Organic Chemistry
Practical DetectionA sample of a compound is 30.9% Na, 21.5% O, and 47.6% Cl by mass.
What is the empirical formula of this compound?
Select the correct answer below:
Organic Chemistry
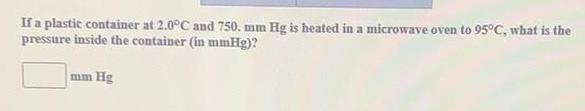
Practical DetectionIf a plastic container at 2.0°C and 750. mm Hg is heated in a microwave oven to 95°C, what is the
pressure inside the container (in mmHg)?
mm Hg

Organic Chemistry
Practical DetectionThe following excerpt is taken from The Scarlet Letter. Do the words explain the narrator's
reaction to the letter "A."
"It seemed to me then, that I experienced a sensation not altogether physical, yet almost so, as of
burning heat, and as if the letter was not of red cloth, but red-hot iron. I shuddered, and
involuntarily let it fall upon the floor."
Which among the following ideas is explicitly stated in the given lines?
Letter A is used as a means to humiliate sinner...
The narrator is made to feel guilty for her sin.
Letter A is Imprinted on the narrator's body.
Letter A is voluntarily worn by the narrator.

Organic Chemistry
Practical DetectionEnter your answer in the provided box.
A volume (39.0 L) of gas at 52 K is heated to 520 K. What volume does the gas (in Liters) now occupy?
(The pressure and the number of particles do not change.)

Organic Chemistry
Practical DetectionEnter your answer in the provided box.
The combustion of coal with oxygen forms CO₂ and releases 94 kcal of energy.
C(s) + O₂(g) → CO₂(g)
How much energy is released when 4.3 mol of O₂ reacts?
ΔH = -94 kcal/mol
kcal of energy released
Report answer to appropriate number of significant figures.

Organic Chemistry
Practical DetectionUpon reading the story, The Scarlet Letter, which among the following was strictly followed in
Puritan society that Hester violated?
1. Illicit affair
II. Refusing to name her child's father
III. Not adhering to all Christian practices
I and II
II and III
I and III
I, II, and III

Organic Chemistry
Practical DetectionDefine Rococo.
A very ornamental style of art that originated in Spain in the early 18th century
A very ornamental style of art that originated in France in the early 16th century
A very ornamental style of art that originated in Austria in the early 14th century
A very ornamental style of art that originated in France in the early 18th century

Organic Chemistry
Practical DetectionWhich terms are examples of musical texture?
monophonic, polyphonic, homophonic
alto, bass, baritone
soprano, mezzo-soprano, contralto
repetition, contrast, variation

Organic Chemistry
Practical DetectionThe cytoplasmic extensions that conduct a received stimulus towards the cell body are called
Schwann cells
dendrons
axons
myelin sheaths

Organic Chemistry
Practical DetectionA compound has a molecular weight of 477.18 g/mol and an empirical formula of C10 H7O2. What is the molecular
formula?
Select the correct answer below:
C30 H2106
C4H1303
C14H1203
none of the above

Organic Chemistry
Practical DetectionEnter your answer in the provided box.
Using the balanced equation for the combustion of ethanol, answer the following question.
C₂H6O(l)+3 O₂(g) → 2 CO₂(g) + 3 H₂O(g)
ethanol
How many grams of CO₂ are formed from 0.70 mol of ethanol?

Organic Chemistry
Practical DetectionBe sure to answer all parts.
MTBE (C5H12O) is a high-octane gasoline additive with a sweet, nauseating odor. Because small
amounts of MTBE have contaminated the drinking water in some towns, it is now banned as a fuel
additive in many areas. MTBE reacts with O₂ to form CO₂ and H₂O. Write a balanced equation for the
combustion of MTBE. Do not included states of matter in your answer.

Organic Chemistry
Practical DetectionBe sure to answer the parts.
How many molecules are contained in each of the following number of moles?
Report your answer using correct significant figures.
a. 0.89 mol of sugar molecules
b. 57.5 mol of acetaminophen molecules