Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
EquilibriumAniline behaves as a weak base When 0 1 M 50 mL solution sample of aniline was mixed with 0 2 M 12 5 mL solution of HCl the pH of resulting solution was 8 Then the correct information is are log 5 0 7 log3 0 48 A pH of 0 01 M solution of anilinium chloride is 5 B pH of original solution of aniline is 3 5 C Upon adding the same aniline sample 50ml 0 1M to the above mixture pH of resulting solution becomes 8 48 D Upon adding the same HCl sample 12 5ml 0 2M to the above mixture pH of resulting solution becomes 4 59

Physical Chemistry
SolutionsCalculate the molarity of a solution prepared by dissolving 12 50 grams of NaNO3 in 250 mL of solution The molar mass of NaNO3 is 85 00 g mol Write your answer with the correct units on the blank below

Physical Chemistry
ElectrochemistryD 2 In argalvanic cell the salt bridge A does not participate chemically in the cell reaction B stops the diffusion of ions from one electrode to another C is necessary for the occurrence of the cell reaction D ensures mixing of the two electrolytic solutions

Physical Chemistry
Equilibrium68 Determine the empirical formula of a compound that contains 39 S 58 5 O and 2 5 H by mass 1 H SO 3 H S O 2 H SO 4 H SO

Physical Chemistry
Chemical Bonding4 Number of optically active isomers 63 Consider the following ALWAY Compound Parama Square planner Synergic gnetism geometry bonding A NiCl 1 B Ni CN 12 C Ni CN D Ni CO X X How many codes are incorrect 1 2 3 3 X 2 1 4 0 X 348482 X 503 OVETRIES 41234 THERE 63 Te alifica A NiC B Ni CN 12 C Ni CN D Ni CO angweile 1 2 3 3 ari zenera Farfacra X ex fandt ants To frefua 2 3d8 2 1 4 0 30 1 1 1 1 1 1 1 1 pane e IUB 03 NIK P3 3olf

Physical Chemistry
Generalgas according to the following equation C8H18 1 2O2 g 8 CO g 9 H O g AH 5074 1 kJ Part A Review Constants What mass of octane in g is required to produce 5250 kJ of heat Express your answer to three significant figures

Physical Chemistry
EquilibriumThe 102 Consider the following reversible reaction of X at an initial concentration X values of the rate constants are k 2 s and k 18 X c b A plot of concentration of X and Y as function of time is a X o X o X o Concentration Concentration Concentration t Meg Xe Yleq Xleq Xleq ea Yea eq

Physical Chemistry
General3 mole of an ideal gas is expanded irreversibly and isothermally at 27 C unit its volume is doubled and 3 5 kJ heat is absorbed form surrounding AStotal system surrounding is 1 20 3 J K 3 40 6 J K 2 20 3 J K 4 40 6 J K

Physical Chemistry
GeneralFrom the following the number of atoms is greater in A 4 g hydrogen C 48 g magnesium One mole of CO2 contains A 6 02 1023 atoms of C B 71 g chlorine D 127 g iodine B 6 02 1023 atoms of O

Physical Chemistry
Electrochemistry0 52 94 The equilibrium constant for the 298 K is Zn s Fe 2 aq Zn given Alum furni 2 aq Fe s Ecell 0 2905 V at 298 K br b 100 595 0 76 d 100 32 0 0295 a e 0 32 0 0295 c 100 0250 0 32 he standard electrode potential for the change a

Physical Chemistry
Chemical Bondingrrect order of strength of H bond H O NH H O NH HF H OX H HF type species are total 10 89 Hef het 1 H O NH3 2 H O NH 3 HF H O 4 NH HF P 0

Physical Chemistry
Atomic StructureIn given figures of FCC unit cell a plane is shown in which certain arrangement of atoms are find here find out number of planes in which given arrangement of atoms can be find iv Match the colum A B C 8 88 ge v Column l Arrangement of the atoms ions 1 2 3 iii Column II Planes in FCC lattice 2 5 4

Physical Chemistry
GeneralWhen 1 4 gm of CaO is added to 98 6 ml of water at 25 C in a calorimeter the temperature of water rises to 30 C Assuming that specific heat of solution is 4 2 J gm C Calorimeter absorbs a negligible amount of heat Calculate AH for the reaction CaO H O 1 Ca OH aq

Physical Chemistry
GeneralA student was carrying out the following chemical reaction in Lab 2A 3B6C balanced reaction He used 30 mole of A and 30 mole of B and at the end of reaction he found 40 moles of C were forme Identify the correct statement s A Total mass before and after the reaction will remain same B B is limiting reagent C yield is 60 D At 50 vield 30 moles of C will be formed

Physical Chemistry
General2 Cu 3 Mn 4 Fe 11 of The number of geometrical isomers CrCl en NH3 where en ethylenediamine is 1 2 2 3 3 4 4 1 12 The total number of geometrical isomers possible for an octahedral complex of the type MA2 B C is M transition metal A B and C are monodentate ligands 1 3 2 4 3 5 4 6 13 The hybridised state of Cu in Cu NH3 4 1 is 1 sp 2 sp 3 sp 4 dsp 14 EAN of Fe in K3 Fe CN 6 is 1 34 2 35 3 36 4 37 15 2 d 3 d 4 d Fill OMR Sheet If above link doesn t work please go to test link fr where you got the pdf and fill OMR from there

Physical Chemistry
Electrochemistry4 Ag Mg Hg Cr K 3 Mg K Ag Hg Cr 4 Ag Mg Hg Cr K 18 The spntaneous redox reaction s among the following is 18 ffald are a 2Fe Fe3Fe 44 b Hg Hg Hg c 3AgCl NO 2H O Given that E Fe Fet EHg Hg 0 77V 1 a 3 a b 0 85 V EAgCV Ag 0 22 V 3Ag 3Cl NO3 4H Efe Fe 0 44 V Eg H92 ENO NO 0 96 V 2 a b c 4 a c 0 92 V a 2Fe Fe b HgT c 3AgCl NO 2H O 2 2 f EO Fe Fet EHg Hg E AgCl Ag 1 a 3 a b Hg 0 77V 0 85 V 3Fe Anod 0 22 V Hg 5 3Ag 3Cl NO3 4H Fe Fe 0 44 V EHgH9 E NO NO 2 a 4 0 92 V A 0 0

Physical Chemistry
Equilibrium4 45 1 575 6 2 287 8 FIAH 47 What amount of energy in KJ is released in the combus 47 11 6 tion of 11 6 gm of C4H10 9 2CH g 130 g 8CO2 g 10H O AH 5756 KJ 2 800 76 3 182 4 57 56 AGJ no On TDS 4 0 11 3qm ey 36 14 AR F 2C4H10 1 575 2 287 3 18 4 57 Het 1 til energy of an electron in Het is 6 8 eV The 48 4 30 9

Physical Chemistry
Solid stateWhich of the following has the highest melting point f fo A Ionic crystal Tafa fenced B Molecular crystal Tufan fanted C Metallic crystal eufraen fonted D Covalent crystal HERING fentent Your Answer A

Physical Chemistry
GeneralA certain metal P has FCC structure with unit cell length 3 16 A the size of largest atom that can be accommodated into the interstices metal without changing the position of metal atoms is O 0 53A O 0 88A O 0 149A None of the above

Physical Chemistry
GeneralE Ecl cr 1 36V E Cr Cr E Cr 0 Cr 1 33V E 0 74V MnO4 Mn b DA to suferit 1 51V 31 sonth Among the following the strongest reducing agent is 1 Cr 3 Cr 2 Mn 4 CI JEE Main 2

Physical Chemistry
Gaseous and liquid statesAt 300 K and 1 atm 15 mL of a gase hydrocarbon requires 375 mL air contai 20 O by volume for complete combust After combustion the gases occupy 330 Assuming that the water formed is in li form and the volumes were measured a same temperature and pressure the form of the hydrocarbon is JEE Main 2016 Off a C3H8 b C4H8 c C4H10 d C3H

Physical Chemistry
Energetics2 1 0 H gas is mixed with air at 25 C under a pressure of 1 atmosphere and exploded in a closed vessel 1 The heat of the reaction H2 g O2 g H O v at constant volume AU298 K 240 60 kJ mol and Cy values for H O vapour and N in the temperature range 298 K and 3200 K are 39 06 JK mol and 26 40 JK mol respectively The explosion temperature under adiabatic conditions is Given N 2 a 2900 K c 2917 K b 2900 C d 3000 C

Physical Chemistry
Electrochemistryc by de How much will the reduction potential of a hydrogen electrode change when its solution initially at pH 0 is neutralised to pH 7 b Decrease by 0 059 V d Decrease by 0 41 V a Increase by 0 059 V c Increase by 0 41 V harge required for the reduction of 1 mole of

Physical Chemistry
Solutions3 At room temperature a dilute solution of urea is prepared by dissolving 0 60 g of urea in 360 g of water If the vapour is 35 mm Hg pressure of pure water at this temperature lowering of vapour pressure will be Molar mass of urea 60 g mol 2019 Main 10 April I

Physical Chemistry
Generalna ing The density of KCI is 1 9893 g cm and the length of a side unit cell is 6 29082 A as determined by X rays diffraction The value of Avogadro s number calculated from these data is 1 6 017 x 1023 2 6 023 x 1023 3 6 03 x 1023 19

Physical Chemistry
Gaseous and liquid states1 In this question you will demonstrate your ability to convert between pressure units use correct significant digits and also to use the gas laws There is a fixed amount of gas in a flexible container Calculate the missing quantity for your line of the table 6 marks App Name Imran Mohamed Olivia Aiden Arlena Jenni Anthony G Kaarunya Nika Seraph Lavigne Leon Lily Linger Anthony M Farhan Madeline Parto Stewart Ujin Aaron Benson Adriana Shiv Sam William Chandler P Torr 150 0 160 0 270 0 280 0 290 0 300 0 310 0 320 0 330 0 340 0 Akkshayaa 350 0 360 0 370 0 380 0 390 0 400 0 410 0 Doris Corwin 170 0 180 0 190 0 200 0 210 0 220 0 230 0 240 0 250 0 260 0 420 0 240 0 180 0 V L 32 0 410 0 0 45 35 5 0 0125 15 0 23 0 4 25 8 5 75 0 22 0 1 65 500 0 2 25 10 5 125 2 80 12 6 225 3 50 14 8 325 4 90 15 5 410 0 7 5 47 5 55 0 75 0 35 5 T C 30 0 25 0 20 0 15 0 10 0 5 0 5 0 10 0 15 0 20 0 25 0 30 0 35 0 40 0 45 0 50 0 55 0 60 0 65 0 70 0 75 0 80 0 85 0 90 0 95 0 100 0 105 0 110 0 20 0 15 0 P2 kPa 50 5 150 0 350 0 200 0 95 0 85 0 110 0 94 0 225 101 155 87 0 120 0 75 0 128 145 78 0 95 0 V L 15 0 0 75 12 5 35 0 49 0 10 0 25 0 0 95 200 0 5 50 75 0 6 0 108 11 4 111 5 6 100 0 105 75 0 25 0 12 5 T C 150 0 25 0 25 5 50 0 100 0 65 0 38 0 40 0 85 0 90 0 160 0 98 0 115 105 50 0 212 45 0 155 0 35 0 38 0 25 5

Physical Chemistry
EquilibriumFor the cell prepared from electrode A and B Electrode A Cr 02 Cr Ered 1 33 V and Electrode B Fe 3 Fe2 Ered 0 77 V Which of the following statements is correct a The electrons will flow from B to A when connection is made 9 b The e m f of the cell will be 0 56 V c A will be positive electrode BB d All of the above 1 33 8 27 cathode High val anode low

Physical Chemistry
GeneralAt t C temperature the observed vapour density of A is 17 5 for the gaseous reaction 2A B 2C If molecular weight of A is 48 then the percentage dissociation of A will be 1 70 27 2 74 28 N

Physical Chemistry
General8 Select correct statement 1 Geometrical isomers of complexes may differ in dipole moment and visible UV spectra 2 Complexes of the type Ma b can also have facial fac and meridional mer isomer 3 No optical isomer exists for the complex trans Co en CL 4 All are correct

Physical Chemistry
Surface chemistry69 The rate of oxidation of oxalic acid by acidified potassium permanganate is shown in the given graph 10 Rate Time 5H C 0 2KMnO 3H SO 2MnSO K SO 10CO 8H O The type of catalyst shown by such reaction is 1 heterogeneous catalysis 2 induced catalysis 3 auto catalysis 4 negative catalysis Chlarinetic

Physical Chemistry
Chemical BondingA buffer solution is made that is 0 317 M in H S and 0 317 M in NaHS If K for H S is 1 00 x 10 7 what is the pH of the buffer solution pH Write the net ionic equation for the reaction that occurs when 0 077 mol HCl is added to 1 00 L of the buffer soluti Use the lowest possible coefficients Omit states of matter Use H O instead of H

Physical Chemistry
ElectrochemistryCopper reduces NO3 into NO and NO2 depending upon the concentration of HNO3 in solution Assuming fixed Cu and PNO PNO the HNO3 concentration at which the thermodynamic tendency for reduction of NO3 into NO and NO2 by copper is same is 10 M The value of 2x is Rounded off to the nearest integer Given E 0 34 V E 0 96 V Cu Cu NO NO RT ENO NO 0 79 V and at 298 K 0 79 V and at 298 K 2 303 F 3 0 0591

Physical Chemistry
Electrochemistryr Q 14 O H S O The product obtained at anode when 50 H SO aqueous solution is electrolysed using platinum electrodes is O H SO3 O 0 4 1 C 00 20

Physical Chemistry
SolutionsAmong 0 1 M solution of urea and Na3PO4 and aluminium sulphate the vapour pressure and freezing point are the highest for urea B the vapour pressure and freezing point are the lowest for urea the elevation in boiling point is the highest for Al2 SO4 3 Dthe depression in freezing point is the highest for Al SO

Physical Chemistry
EquilibriumWhich of the following is incorrect 1 Solid equilibrium 2 17 90 60 liquid both phase exist only at one value of temperature 2 Liquid vapour both phase may exist equilibrium at a given temperature and pressure 3 Solubility of solid in liquid remain same at given temperature 4 The solubility of gas in a liquid is directly proportional to partial pressure of the gas present above the surface of the liquid

Physical Chemistry
Atomic Structureder the following series of reactions Cl NaOH NaCl NaClO H O NaClO NaCl NaClO NaCIO NaClO NaCl How many moles of NaCIO are obtained after the completion of reactions by taking 1 m Cl NaOH in excess A mol B Zero c mo C D None of these

Physical Chemistry
Gaseous and liquid states3 A mixture weighing 228 g contain CaCl and NaCl If this mixture is dissolved in 10 kg of water and form ideal solution that boil at 100 364 C The mol of NaCl in mixture is K of water 0 52 K mol kg 1 33 3 3 50 2 66 67 4 75

Physical Chemistry
GeneralThe atom represents 1 It is an atom of fluorine II The atomic number of fluorine is 9 III The nucleus of fluorine has 9 protons IV There are 9 electrons revolving around the nucleus of fluorine 6 719

Physical Chemistry
GeneralDOMY 3 A second reaction mixture was made up in the following way 10 mL of 3 0M acetone 10 mL of 0 0030M 1 5 0 mL of 1 5M HCI 25 mL H O a Calculate the molarities of acetone H ion and I in the reaction mixture

Physical Chemistry
EquilibriumpH of the resultant solution made by mixing 500 mL of 0 2 M NH4OH with 500 mL of 0 1 M HCI solution is pKb NH4OH 4 7 4 7 3 4 10 6

Physical Chemistry
GeneralConsider the salt NH4F in which the weak acid HF and the weak base NH4OH have different strengths 1 8 x 10 5 In such solutions since H OH the pH of such i e Ka of acid 7 2 x 10 4 and Kb solutions less than 7 acidic Case 3 If Ka Kb pH 7 Consider the salt NH4CN in which the weak acid HCN and the weak base NH4OH have differen strengths i e Ka of acid 4 0 x 10 10 and Kb 1 8 x 10 5 In such solutions since H OH hence t pH of such solutions more than 7 basic

Physical Chemistry
Gaseous and liquid statesKm KT 22 One half mole each of nitrogen oxygen and carbon dioxide are mixed in enclosure of volume 5 litres and temperature 27 C The pressure exerted by mixture is R 8 31 mol K a 7 48 x 105 Nm b 5x105 Nm c 6 x 105 Nm d 3 x 10 Nm 23 From a certain apparatus the diffusion rate of
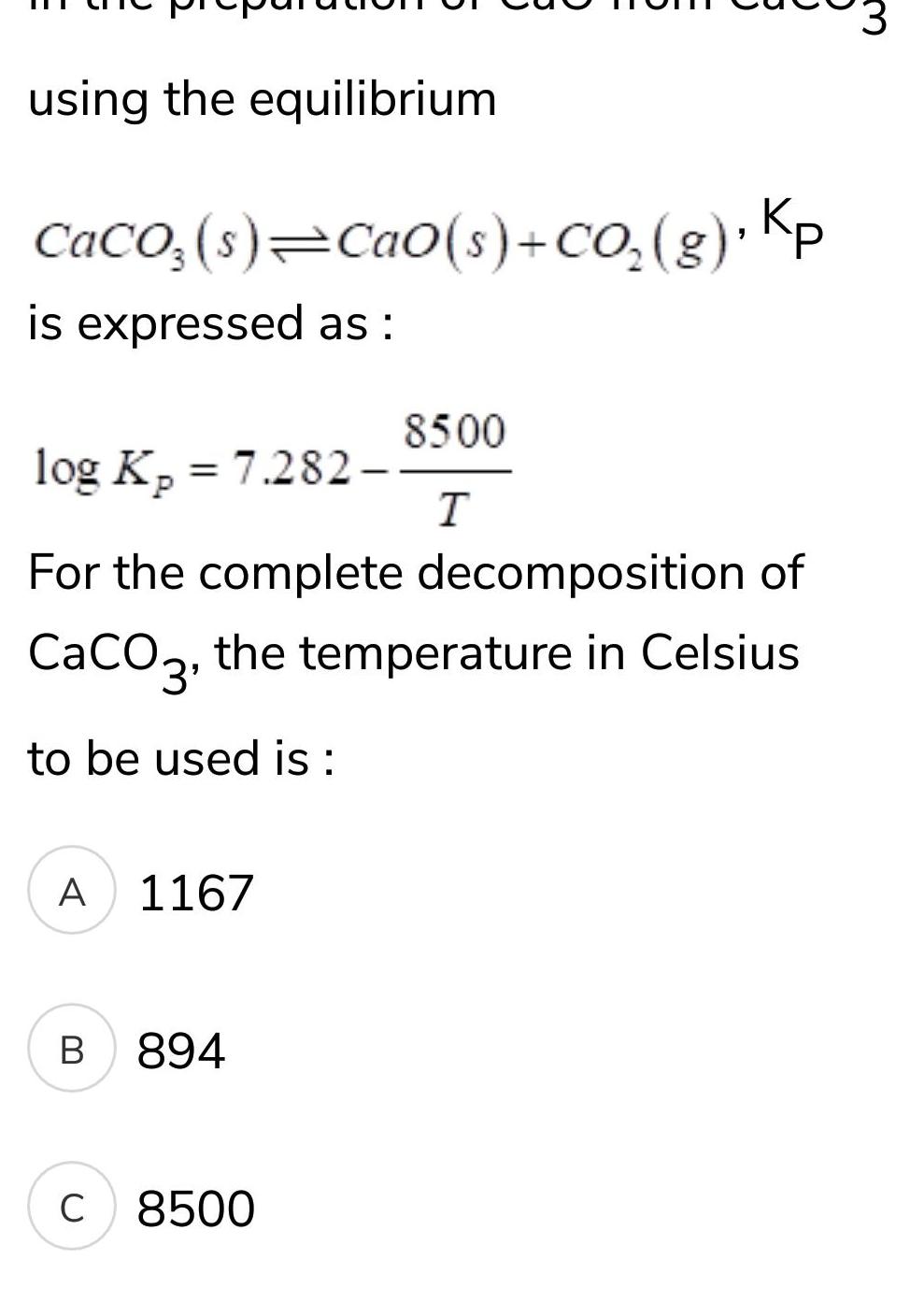
Physical Chemistry
Gaseous and liquid statesusing the equilibrium CaCO3 s Ca0 s CO g Kp is expressed as log K 7 282 Kp For the complete decomposition of CaCO3 the temperature in Celsius to be used is A 1167 B 894 8500 T C 8500

Physical Chemistry
Nuclear chemistryConsider the following nuclear reactions involving X Y X Y He Y 0 8 H If both neutrons as well as protons in both the sides are conserved in uclear reaction then moles of neutron 4 6 gm of X A 2 4 NA C 4 6 B 2 4 D 0 2 NA

Physical Chemistry
General33 Which one of these graphs for an ideal gas the arrow indication is incorrectly marked decreasing Press a c 0 P 0 decreasing Volume Temp in K Increasing Temp 2009 2 noolled ST101 b 273 d P 1 V 1 C decreasing Press

Physical Chemistry
GeneralExperiment P you titrated a weak acid with a strong base When 1 336 g of an unknown acid HA was dissolved in 100 0 mL of water and titrated with 0 492 M NaOH the value of pH was 2 70 after 0 00 mL of NaOH were added and was 70 pka after 13 29 mL were added The molar mass of the acid HA is in g mol 204 106

Physical Chemistry
Solutionsc 1500 3 3 An ideal solution was obtained by mixing methanol and ethanol If the partial vapour pressure of methanol and ethanol are 2 619 kPa and 4 556 kPa respectively the composition of vapour in terms of mole fraction will be a 0 635 MeOH 0 365 EtOH b 0 365 MeOH 0 635 EtOH c 0 574 MeOH 0 326 EtOH H0827 EtOH

Physical Chemistry
SolutionsIf Ksp of the salt A3B is 2 7 x 10 11 then solubility of the salt in mol L will be 2 7 x 10 3 M 2 7 x 10 4 M 10 3 M 10 4 M

Physical Chemistry
ElectrochemistryIf 0 50 L of a 0 60 M SnSO4 solution is electrolyzed for a period of 30 0 min using a current of 4 60 A If inert electrodes are used what is the final concentration of Sn remaining in the solution at wt of Sn 119 1 0 342 M 2 0 6 M 3 0 389 M 4 0 514 M

Physical Chemistry
ElectrochemistryA current of 2 ampere is passed through copper and silver voltameters connected in series If 1 08 gm silver equivalent weight 108 is deposited on cathode in silver voltameter then find the amount of copper deposited at cathode in copper voltameter Given equivalent weight of Cu 31 8