Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
GeneralFor the reaction A B Product following data are given Expt No A 1 1M 1M 2 2M 1M 3 2M 2M Order of reaction will be B C D 1 2 3 A o 2 5 B o Rate M Sec 1 1 2 8

Physical Chemistry
Surface chemistrySelect the incorrect option s amongst the following A Gold sol is a positive charged sol B Lyophobic sols can act as protective colloid for Lyophilic sols C When FeCl is hydrolysed with hot water then ferric hydroxide sol positiv charged will be obtained D Colour of colloidal solution depends on size shape of dispersed phase

Physical Chemistry
EquilibriumAn aqueous solution of 6 3 g of oxalic acid dihydrate is made up of to 250 ml The volume of 0 1 N NaOH required to completely neutralise 10 ml of this solution is A 40 ml C 10 ml B 20 ml D 4 ml

Physical Chemistry
Nuclear chemistryfor f 18 The kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is a is Bohr 2h Given as then x 2 2 x maz

Physical Chemistry
Electrochemistry39 What will be the molar conductivity of Al ions at infinite dilution if molar conductivity of Al SO is 858 Scm mol and ionic conductance of SO2 is 160 Scm mol at infinite dilution a 189 Scm mol b 698 Scm mol c 1018 Scm mol d 429 Scm mol

Physical Chemistry
Atomic StructureWhich of the following statement is INCORRECT Li is harder than the other alkali metals In Solvay process NH3 is recovered when the solution containing NH CI is treated with H O Na CO is pearl ash Beryllium and aluminium ions do not have strong tendency to form complexes like ReF

Physical Chemistry
Chemical BondingAmong the following pairs of species in which first species has lower ionisation energy and higher electron affinity than second species 1 N and O 11 O and S 11 Be and B v Li and Lit iv Cl and Cl vi F and Cl

Physical Chemistry
General52 Mark out the correct statement among the following 1 Aqueous AgNO solution can be stored in a copper bowl 2 Aqueous CuSO solution can be stored in a silver bowl 3 Cu Ag can release hydrogen gas from dil HCI 4 H cannot reduce Cu and Ag in the form of metals 53 In the reaction

Physical Chemistry
Chemical kineticsIn a first order reaction the concentration 64 of the reactant decreases from 800 mol dm to 50 mol dm in 2 x 104 sec The rate constant of the reaction in s is 1 2 x 104 2 3 45 x 10 5 43 1 386 10 4 3 10 K 2 303 log t log 2 303 2x104 P 13 BG

Physical Chemistry
GeneralDecomposition of 3A g 2B g 2C g follows 1st order kinetics Initially only A is present in the container Pressure developed after 20 min and infinite time are 3 5 and 4 atm respectively Which of the given option s is are correct A 50 20 min B 175 40 min C 199 64 3min D 87 5 60 min

Physical Chemistry
ElectrochemistryWater is reduced to H at 298 K according to the given reaction H O e 1 H g OH aq The reduction potential of the above half cell at standard state is x V then the value of 10 x is where represents greatest integer function Use 2 303RT F 0 059 and K 10 4 at 298 K Answer 0 1 2 3 4 5 6 7

Physical Chemistry
Solutions15 What volume of 0 1 Noxalic acid solution can be reduced by 250 g of an 8 percent by weight KMnO solution a 6 3 litre b 12 6 litre c 25 2 litre d 0 63 litre

Physical Chemistry
SolutionsA liquid Avapour 30 Which of the following azeotropes is not correctly matched a HNO 68 H O 32 Maximum boiling azeotrope B P 393 5 K b H O 43 HI 56 7 Minimum boiling azeotrope B P 290 K c C H OH 95 5 H O 4 5 Minimum boiling azeotrope B P 351 15 K d Chloroform 93 2 C H OH 6 8 Minimum boiling azeotrope B P 332 3 K

Physical Chemistry
GeneralWhen x g carbon is burnt with y g of oxygen in a closed box and no residue is left which of following statement is correct regarding the relative amounts of oxygen and carbon must be less than 1 33 a c X y X must be greater than 2 67 y b must be greater than 1 33 X d y must lie between 1 33 and 2 67 X

Physical Chemistry
Chemical kineticsWhich of the following is correct A B C D larger the activation energy high will be the value of temperature coefficient of reaction with increase in temperature in high temperature range k will increase more compare to low temperature range 1 Graph of k vs is a straight line with slope T Ea 2 303R Chemical reaction with low activation energy always occurs with fast rate compare to reaction with high activation energy

Physical Chemistry
Solid stateal lattice cture to high pressure tions of positive and tice The density of crystalline sodium chloride is 78 5 85 g cm 3 What is the edge length of the unit cell 14 06 x 10 8 cm 2 1 32 x 10 14 cm 4 9 6 x 10 24 3 7 8 x 10 23 A unit cell of sodium chloride has four formula units 79

Physical Chemistry
Equilibrium0 5 mole of HCI and 1 0 mole of CH3COONa are added to water and the volume was made up to 1 litre What is the H ion concentration of the resultant solution Ka for CH3COOH is 1 6 10 5 A B C D 4 6 x 10 5 4 x 10 3 0 0042 1 6 x 10 5

Physical Chemistry
SolutionsOn increasing dilution of electrolytic solution A B C D Equivalent conductance decreases Molar conductance increases Specific conductance increases All of the above

Physical Chemistry
Solid statec 4 d 5 19 If a crystal of LiCl has two F centre sites per unit cell then which of the following cannot be the ratio of number of effective Lit number of effective Cl ions Assuming NaCl type structure a 1 33 b 1 06 c 2 b d 1 18

Physical Chemistry
Solid stated 415 pm 39 Edge length of unit cell of Chromium metal is 287 pm with bcc arrangement The atomic radius is the order of a 124 27 pm b 287 pm c 574 pm d 143 5 pm D In the ionic comu

Physical Chemistry
Generald 9 14 A 0 1097 g sample of As O required 36 10 mL of KMnO solution for its titration The molarity of KMnO solution is a 0 02 M c 0 0122 M 15 0 52 g of a dibasic acid required 100 ml b 0 04 M d 0 3 M NaOH

Physical Chemistry
SolutionsC L 53 Dissolution of 1 5 g of a non volatile solute molecula weight 60 in 250 g of a solvent reduces its freezin point by 0 01 C Find the molal depression constant c the solvent a 0 01 c 0 0001 b 0 001 d 0 1

Physical Chemistry
General14 If AgCl is doped with 105 mol of CdC1 The concentration of cation vacancies is b 6 02 x 1018 d 6 02 x 1016 a 6 02 1028 c 6 02 x 1017 5 An element at wt 50 crystallises in fcc lattice with a 0 50 nm What is the density of unit noll if it

Physical Chemistry
Solutions66 The Van t Hoff factor of 0 005 M aqueous solution of KCI is 1 95 The degree of ionisation of KCl is a 0 95 b 0 97 d 0 96 c 0 94 67 02 molal ag HA acid ionises to the extent of 20 V

Physical Chemistry
Generald All are correct 13 When Cr s OH aq Cr OH aq H g basic solution is balanced the sum of the coefficients of all the reactants and products is a 14 b 15 c 17 d 9 14 A 1097 a samplo

Physical Chemistry
General60 In the following redox reaction XUO Cr O yHxUO2 zCr 6H O the value of coefficients x y and z respectively are b 3 8 7 d 3 1 8 a 3 8 2 c 3 2 4 How many moles of iodine are liberated wit

Physical Chemistry
Chemical Bondingb Textile substrate is dyed using three colours Write down the formula for K M function for this dyed sample Explain each term c Name triadic colour arrangements which can be used to generate colour schemes

Physical Chemistry
GeneralIf O concentration in tissue is almost as higher as at the respiratory surface A B D Oxyhaemoglobin would not dissociate to supply O to the tissue CO will interfere the O transport Oxyhaemoglobin would dissociate to supply O to the tissue Haemoglobin would combine with more O at respiratory surface

Physical Chemistry
Equilibriumis 8 x 10 cm Hence Ksp of BaSO4 is 4x 10 M 2 1 x 10 M 3 2 10 M K 8X10 K eq 400 M 8 105

Physical Chemistry
SolutionsThe freezing point of a 4 w v aqueous solution of A is equal to the freezing point of 10 w v aqueous solution of B The molecular weight of A is 60 find out the molecular weight of B Assume both A and B are non electrolyte and molarity molality 1 150 3 45 2 90 4 180

Physical Chemistry
Gaseous and liquid statesFor a real gas obeying van der waals equation which of the following is are true A Internal energy of gas is dependent on temperature only B If Z 1 then forces of repulsion are dominant C At very high pressure for most of the gases Z 1 1 RD RT D The second virial coefficient B depends on the nature of gas and temperature

Physical Chemistry
Chemical BondingA 20 gm mixture of isobutane and isobutene requires 40 gm of Br in CCl4 for complete addition this mixture is catalytically hydrogenated and the entire alkane is monobrominated in the presence o light at 127 C how much of product is produced At Wt of bromine 80

Physical Chemistry
Solid stateIn gravimetric analysis the ideal product should be very pure insoluble easily filterable and should possess a known composition very pure soluble and should possess a known composition very impure soluble and should possess a known composition pure insoluble easib able and should possess an unknown composition

Physical Chemistry
ElectrochemistryConsider the following solutions Solution P aq solution of Cu NO3 2 Solution Q aq solution of AgNO3 Solution R conc aq solution of NaCl Solution S aq solution of KNO3 If electrolysis of these solutions takes place using in electrodes in different containers Among the following set of the solution s in which pH does not increase due to electrolysis is are assume no volum change due to electrolysis

Physical Chemistry
Surface chemistryWhich of the following is incorrect match A B C physisorption multilayered Active Adsorption Requires activation energy Lyophobic colloid charge is present

Physical Chemistry
General2 An atomic solid crystallizes in a body centre cubic lattice and the inner surface of the atoms at the adjacent corner are separated by 60 3 pm If the atomic weight of A is 48 then density of the solid is nearly b 5 07 g cc a 2 7 g cc c 3 5 g cc d 1 75 g cc

Physical Chemistry
Solid stateEfficiency 74 41 If the distance between Nat and Cl in NaCl crystal is 265 pm the edge of the unit cell will be a 265 pm b 795 pm c 132 5 pm d 530 pm 42 Sodium metal crystallises in bodu 2 2r Packing

Physical Chemistry
Solid state1 AB 2 A B 4 3 A B3 4 A zB n A FCC crystal of O 2 oxide ions has Mtn ions in 50 of octahedral voids then the value of n is 1 2 2 3 4374 4 1 If the edge length of a KCl unit cell is 488 pm what is the length of KCl bond if it crystallises in the FCC

Physical Chemistry
Solid stated A Y 2 21 If in a crystal lattice of a compound each corner of cube is enjoyed by sodium each edge of a cube has oxygen and centre of cube is enjoyed by Tungsten W then give its formula a Na WO4 c Na WO3 b NaWO d Na WO

Physical Chemistry
Solutionsa 0 85 C c 0 C b 3 53 C d 0 35 C 75 Maximum lowering of vapour pressure is observed in the case of a 0 1 M glucose c 0 1 M MgSO b 0 1 M BaCl d 0 1 M NaCl

Physical Chemistry
Gaseous and liquid states9 A Mixture of Cyclopropane gas and oxygen is used as an anesthetic cyclopropane containes 85 7 C and 14 3 H by mass At 50 C and 0 984 atm pressure 1 56 g cyclopropane has a volume of 1 00 L what is the molecular formula of cyclopropane

Physical Chemistry
Electrochemistryc 390 2 Resistance of a conductivity cell filled with a solution of electrolyte of concentration 0 1 M is 100 2 The conductivity of this solution is 1 29 S m Resistance of the same cell when filled with 0 2 M of the same solution an

Physical Chemistry
Solid state2 The simplest formula of a solid having CCP arrangement for A atoms in which alternate face centres are occupied by B atoms and alternate edge centres are occupied by C atoms is a ABC c A BC b A BC d A B C

Physical Chemistry
GeneralQUESTION 23 Chemometrics is the science of extracting information from measurements made on MARKS 1 chemical systems physical system hypothetical system natural system

Physical Chemistry
ElectrochemistryThe Ksp of Ag2 CrO4 AgCl AgBr and Agl are respectively 1 1 x 10 12 1 8 10 10 5 0 x10 13 8 3 x 10 17 Which one of the following salts will precipitate last if AgNO3 solution is added to the solution containing equal moles of NaCl NaBr Nal and Na CrO4 1 AgCl 2 AgBr 3 Ag CrO4 4 Agl

Physical Chemistry
Chemical kineticsFor a reaction 2B products 6000 T log k M hr 20 life period is assuming initial conc of B as 1M 1 6 93 x 10 21 hr 2 1 15 x 10 4 hr 3 5 x 10 21 hr 4 1 x 10 20 hr The lower limit of ha
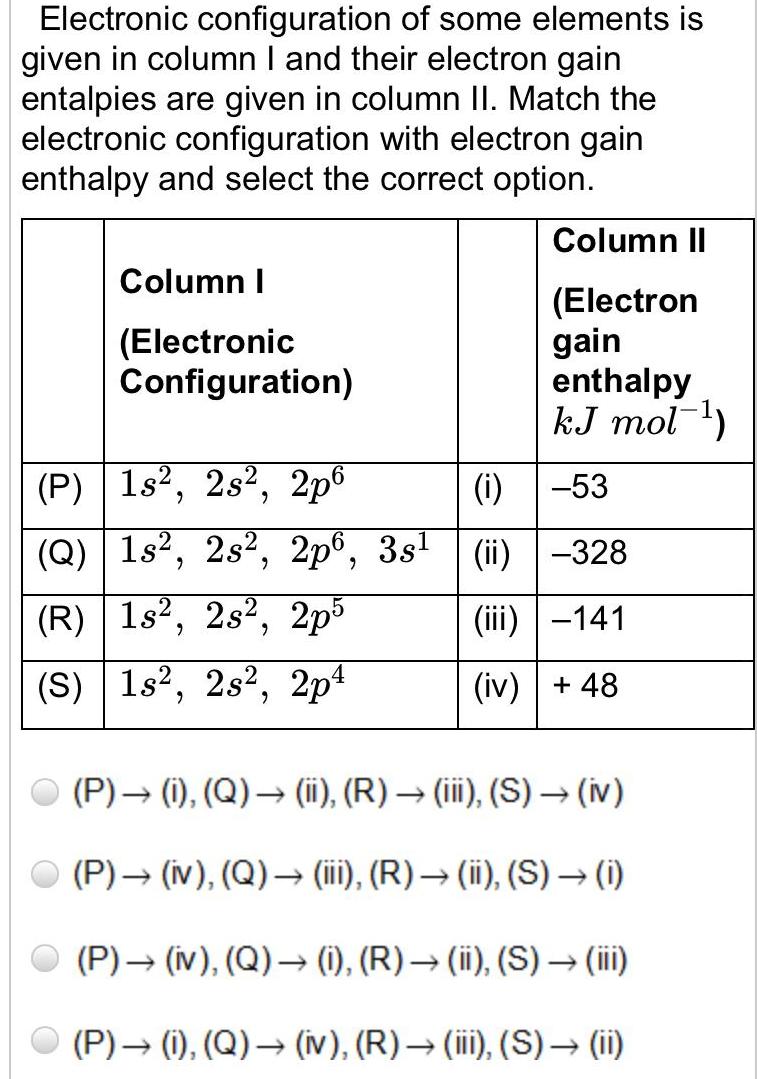
Physical Chemistry
Atomic StructureElectronic configuration of some elements is given in column I and their electron gain entalpies are given in column II Match the electronic configuration with electron gain enthalpy and select the correct option Column I Electronic Configuration P 1s 2s 2p6 Q 1s 2s 2p6 3s R 1s2 2s2 2p5 S 1s2 2s2 2p4 Column II Electron gain enthalpy kJ mol i 53 ii 328 iii 141 iv 48 P i Q ii R iii S iv P iv Q iii R ii S i P iv Q i R ii S iii P i Q iv R iii S ii

Physical Chemistry
Equilibrium5 Assertion pH of a buffer solution does not change on dilution Reason On dilution the ratio of concentration of salt and acid or base remains unchanged

Physical Chemistry
Chemical kineticsA rise of 10 C can cause the rate of some reactions to double This is best explained by A B C D The average velocity of the molecules has doubled The number of molecules with more than enough energy to overcome the activation energy barrier has doubled The activation energy has lowered The average kinetic energy of the particles has doubled

Physical Chemistry
Solid stateCABC d ABCAABCA 26 Salt AB has a zinc blende structure The radius of A2 and B ions are 0 7 and 1 8 respectively The edge length of AB unit cell is a 2 5 b 5 09 A c 5 d 5 77 C