Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Chemical kineticsOn electrolysis of dil sulphuric acid using Platinum Pt electrode the product obtained at anode will be 1 Hydrogen gas Oxygen gas 3 4 H S gas SO gas Gigon 4 has 78 And

Physical Chemistry
General26 The absolute configuration of the following compound is H CI a 2 S 3 R c 2 B 35 CH 2 Cl H 3 C H b 2S 3S d 2 R 3 R 2003

Physical Chemistry
SolutionsTwo bottles of A and B contains 1M and 1m aqueous solution d 1g mL of sulphuric acid respectively 1 A is more concentrated than B 2 B is more concentrated than A 3 Concentration of A conc of Bed 4 It is not possible to compare the entration

Physical Chemistry
Solid stateS No Radius ratio 1 2 3 4 5 6 small large Geometric shape of the crystal formed upto 0 15 Linear 0 15 to 0 22 Trigonal planar 0 22 to 0 41 Tetrahedral 0 41 to 0 73 Square planar 0 41 to 0 73 Octahedral 0 73 to 0 99 Cubic Co ordi nation number of the ion 2 3 4 4 6 8

Physical Chemistry
Gaseous and liquid statesWhat is the variation of Z with pressure a At very low pressures all gases show Z 1 At high pressures all gases show Z 1 b c At intermediate pressures most gases show Z 1 d All of the above
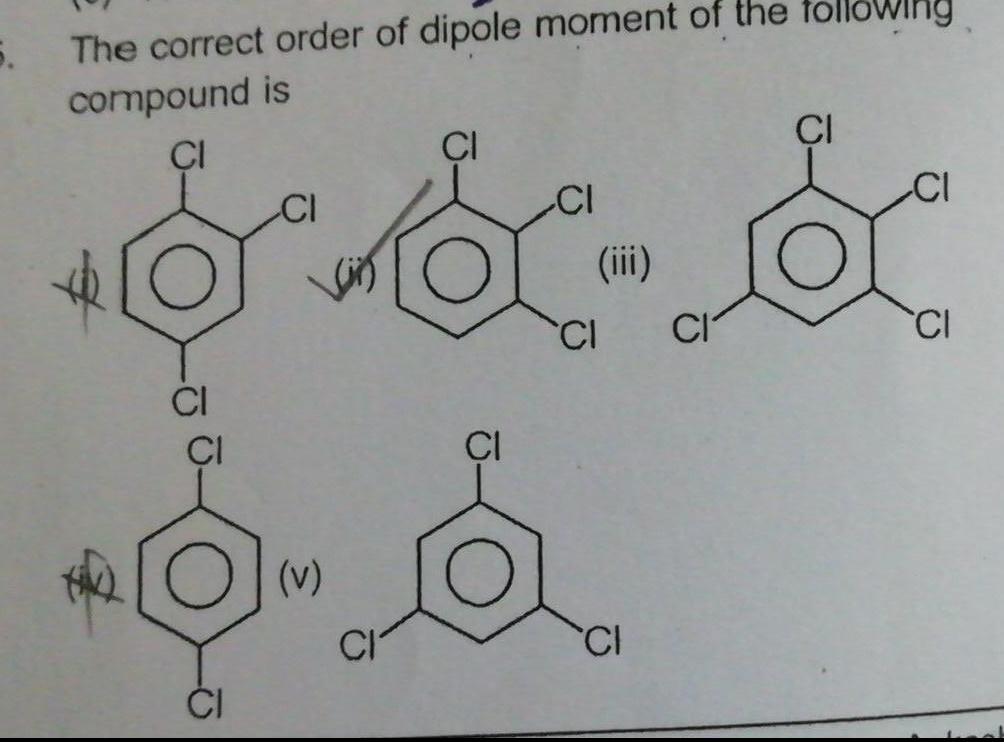
Physical Chemistry
General5 The correct order of dipole moment of the compound is CI CI CI v CI CI iii CI CI CI owing CI

Physical Chemistry
ElectrochemistrySmall spherical ball of silver metal used in jewellery having diameter 0 1 cm which is obtained by the electrolytic deposition It total number of balls in jewellery is 10 000 then calculate the applied amount of electricity in coulombs which is used on the deposition on electrodes having entire surface 0 12 m Density of Ag 10 5 It is assumed that 3 5 electricity consumed as wastage during electrolysis and 60 of electrode body immersed in electrolyte Give your answer in multiple of 104

Physical Chemistry
EquilibriumQ 4 95 B H O C NO3 Out of the following amphiprotic species in aqueous medium are I HPO II OH H PO A I III IV B I and III C III and IV When ammonia is added to water it decreases the concentration D H O and NO3 IV D All of which of HCO

Physical Chemistry
GeneralWhich of the following is most likely formula of platinum complex if of total chlorine 4 of the compound is precipitated by adding AgNO to its aqueous solution 1 PtCl 6H 0 2 PtCl4 5H O 3 PtCl 2H O 4 PtCl 3

Physical Chemistry
Solutions200 ml of a sample of water required 2 94 mg of K Cr O eq wt 49 in the presence of H SO for the oxidation of dissolved organic matter in it The COD of the water sample is a 2 4 ppm b 4 8 ppm c 9 6 ppm d 16 ppm

Physical Chemistry
SolutionsAIMS 2017 0 5g of an unknown solute is dissolved in 295 g solvent If molarity and density of solution are 47 0 05 M and 1 5 g cc respectively The molecular weight of unknown solute is 1 375 2 425 3 400 The depression in freezing poi Molality 4 500

Physical Chemistry
General44 gram sample of a natural gas consisting of methane CH and ethylene C H was burned in excess of oxygen yielding 132 gm CO and some H O as products What is the mole of ethylene in the sample

Physical Chemistry
GeneralE A student used a carbon pencil to write his homework The mass of this was found to be 5 mg With the help of this calculate a The number of moles of carbon in his

Physical Chemistry
Atomic Structure30 Calculate the wavelength and energy of radiatio emitted for the electronic transition from infinity to the stationary state of the hydroge atom

Physical Chemistry
Gaseous and liquid statesA 100 0g ice cube at 0 0 C is placed in 650 of water at 25 C what is the final temperature of the mixture 1 11 C 2 12 5 C 3 18 C 4 16 C

Physical Chemistry
General586 Which of the following concentration of metals in drinking water is not prescribed 1 Fe 0 2 ppm 2 Cu3 0 ppm 5 3 Cd 2 0 ppm 4 Zn 5 0 ppm 880

Physical Chemistry
Atomic Structure36 The following transition occurs when Lithium atoms are sprayed into hot flame The various steps are numbered for identification 2s 2p 3d Which of these transitions result in emission of light A I II V B III V III 3p IV 4s 3p C III IV V D All

Physical Chemistry
GeneralD Add the suffixes en ful less y ly tal able al ive or ous to make adjectives You might get more than one adjective from some words Watch your spellings NOUNS gold wood 3 silk 4 skill 5 care 6 cheer 7 power 8 coward 9 love 10 live 11 friend 12 remark 13 measure 14 response 15 appreciate ADJECTIVES golden silhey skillly Lovely the livey Sriendly desporbely abbrevilaty NOUNS 16 attract 17 elude 18 taste 19 storm 20 star 21 sleep 22 cream 23 powder 24 length 25 hand 26 bulb 27 ridicule 28 horizon 29 element 30 region ADJECTIVES part starry Speeb cream powse longth hand bulls ridicale 0001201 element TIO

Physical Chemistry
GeneralUsing the following Latimer diagram for bromine 7 pH 0 BrO4 1 82 V Bro 1 50 V 7 8 HBrO 1 595 V 1 06552 V Br the species undergoing disproportionation is a BrO4 b BrO3 c HBrO d Br Br

Physical Chemistry
Gaseous and liquid statesVolume of the flask in which species are transferred is double of the earlier flask In which of the following cases equilibrium is affected 2 Ens LO I N g 3H g 2NH g HO II N g O g 2NO g III PC1 g PC1 g Cl g HO IV 2NO g N g O g 1 I II 2 II III 3 I III 1 4 III IV

Physical Chemistry
Gaseous and liquid statesAn open vessel at 27 C is heated until 3 5 parts of air in it has been expelled Assuming that the volume of the vessel remains constant find the temperature to which the vesel had been heated Ans 750K

Physical Chemistry
SolutionsThe mass of glucose that should be dissolved in 50 g of water in order to produce the same lowering of vapour pressure as is produced by dissolving 1 g of urea in the same quantity of water is a 1 g c 6 g b 3g d 18 g 2006

Physical Chemistry
EquilibriumD At 27 C N2O4 g dissociates 40 into NO2 g the equilibrium vapour density of the mixture is 1 32 85 2 40 26 3 46 4 72 41

Physical Chemistry
EnergeticsWhen 1 mole of H O2 is decomposed by platinum black the heat evolved is 96 6 kJ The heat of formation of 1 mole of H O2 is a 96 6 kJ c 386 4 kJ b 193 2 kJ d 48 3 kJ

Physical Chemistry
Gaseous and liquid statesA sample of a hydrate of barium chloride weighing 61 g was heated until all the water of hydration is removed The dried sample weighed 52 g The formula of the hydrated salt is atomic mass Ba 137 amu Cl 35 5 amu 1 BaCl H O 2 BaCl 2H O 3 BaCl 3H O 4 BaCl 4H O
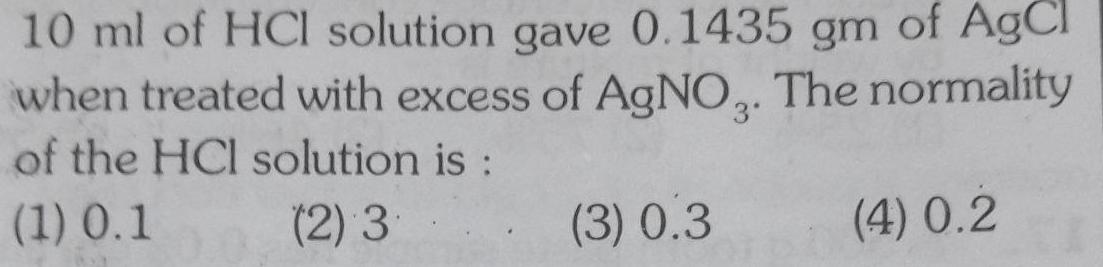
Physical Chemistry
Solutions10 ml of HCl solution gave 0 1435 gm of AgCl when treated with excess of AgNO3 The normality of the HCl solution is 1 0 1 2 3 3 0 3 4 0 2

Physical Chemistry
General500 ml solution of 1M CaBr solution d 1 2 gm ml is mixed with 500 ml of 1m Nal solutio d 1 15 gm ml Choose the correct statements regarding resulting solution At wt of Br 80 I 127 Ca 40 Molarity of CaBr in resulting solution is 0 5M BMolality of Nal in resulting solution is 0 5M Volume of resulting solution is 1000 ml D Density of resulting solution is 1000 kg m

Physical Chemistry
Gaseous and liquid statesFor the reaction N g 3H g 2NH g Heat of reaction at constant volume exceeds the heat of reaction at constant pressure by XRT The value of x is

Physical Chemistry
SolutionsSolvent which is better to be used during ebullioscopic measurement has 1 High K 3 K K 2 Low K 4 Unpredictable

Physical Chemistry
Surface chemistryMedicines are more effective if they are used in 2 Solid state 4 All of the above 1 Colloidal state 3 Granular state

Physical Chemistry
Energetics6 The species which by definition has zero standard molar 2010 enthalpy of formation at 298 K is a Br g c H O g The bond Taal b Cl g d CH g

Physical Chemistry
Equilibrium0 9 What would be the pH of a solution obtained by mixing 5 g of acetic acid and 7 5 g of sodium acetate and making the volume equal to 500 mL JEE MAIN Online 2013 Ka 1 75 x 10 5 pKa 4 76 1 4 76 pH 5 0 3 pH of solution will be equal to pH of acetic acid 2 pH 4 70 4 pH 4 70

Physical Chemistry
General4 To 50 litre of 0 2N NaOH 5 litre of 1N HCl and 15 litre of 0 1N FeCl3 solution are added What weight of Fe 03 can be obtained from the precipitate Also report the normality of NaOH left in the resultant solution

Physical Chemistry
Generalc 5x10 kg 5 The number of atoms in 4 25 g NH3 is approximately b 1 5x 1023 roles as d 6 1023 orn atomic weight scale is based on a 1x 1023 c 2 1023

Physical Chemistry
EquilibriumFrom separate solutions of four sodium salts Naw NaX NaY and NaZ had pH 7 0 9 0 10 0 and 11 0 respectively when each solution was 0 1 M the weakest acid is 1 HW Hol 2 HX XZ 4 HZ

Physical Chemistry
General2 The wavelength of the first line of the He ion spectral series whose interval between the extreme lines is 2 725 x 106 m is R 1 09 107 m a 471 82 nm c 1019 37 nm b 4718 2 nm d 165 14 nm

Physical Chemistry
Atomic Structure21 Find the quantum no n corresponding to excited state of He ion if on transition to the gr state that ion emits two photons in succession wavelengths 108 5 and 30 4nm

Physical Chemistry
Solutions26 The molecular weight of benzoic acid in benzene as determined by depression in freezing point method corresponds to 1 Ionisation of benzoic acid 2 Dimerization of benzoic acid 3 Trimerization of benzoic acid 4 Solvation of benzoic acid

Physical Chemistry
Atomic Structure3 n p d bond 4 Three p d bonds Bond distance in HF is 9 17 x 10 11 m Dipole moment of HF is 6 104 x 10 30 Cm The percent ionic character in HF will be electron charge 1 60 x 10 9 C JEE MAINS 2013 On line 3 35 5 1 61 0 2 38 0 4 41 5 The shape of IF is JEE MAINS 2013 On line

Physical Chemistry
Equilibriumof a gas is found to be 5 46 g dm at 27 C at 2 bar pressure What will be its density at STP

Physical Chemistry
General3 100 mL of O gas diffuses in 10 s 100 mL of gas X diffuses in t sec Gas X and time t can be 1 H 2 5 s 2 SO2 16 s 3 CO 10 s 4 He 4 s

Physical Chemistry
GeneralAL 2017 QUILIBRIUM 3 PHYSICAL CHEMISTRY Reaction N O NO NO was studied by taking 2 mol L of N O initially A equilibrium degree of dissociation of N O was found to be 10 What is the ratio of equilibrium concentration of N O NO and NO 1 19 1 1 3 1 1 9 The equilibrium H g CO g 2 9 1 1 4 18 1 1 constant for reaction H O g CO g is 1 8 at 1000 C If one mole of H and 1 0 mole of CO are placed in a one litre flask The final

Physical Chemistry
Solid stateWhich is the incorrect statement a FeO0 98 has non stoichiometric deficiency defect b Density decreases in case of crystals with Schottky s defect c NaCl s is insulator silicon is semiconductor silver is conductor quartz is piezoelectric crystal d Frenkel defect is favoured in those ionic compounds in which sizes of cation and anions are almost equal NEET 2017 metal

Physical Chemistry
GeneralA particle executes SHM of amplitude A i At what distance from the mean position is its kineti energy equal to potential energy ii At what points is its speed half the maximum speed

Physical Chemistry
Solid stateThe number of octahedral void s per atom present in a cubic close packed structure is 1 2 3 1 2 4 4 3 AIPMT 2012

Physical Chemistry
Energetics1 mole of methanol when burnt in oxygen gives out 723 kJ mol heat If 1 mole of oxygen i used what will be the amount of heat evolved 2 964 kJ 1 723 kJ Ans 3 3 482 kJ 4 241 kJ

Physical Chemistry
EquilibriumMixture of ester and HCl is titrated with NaOH using phenolphthalein as an acidic indicator at end point pink color dissappear after some time due to 1 CH COOH is formed 2 Due to weak acedic nature of CH OH 3 Regeneration of HCI LO 4 Due to ionization of phenolphthalein

Physical Chemistry
Gaseous and liquid states41 The molal boiling point constant for water is 0 513 C kg mol When 0 1 mole of sugar is dissolved in 200 ml of water the solution boils under a pressure of one atmosphere at 2011 a 100 513 C c 100 256 C b 100 0513 C d 101 025 C

Physical Chemistry
SolutionsSingle The vapour pressure of pure benzene at 25 C is 639 7 mm Hg and the vapour pressure of solution of solute in benzene at 25 C is 631 9 mm Hg The molality of the solution is 7 Types A 0 156 C 0 518 B 0 108 D 0 815

Physical Chemistry
Surface chemistry0 X Graph between log and log p is a straight line at an angle 45 with intercept on y axis 0 3010 of Calculate the amount of gas adsorbed in gram per gram of the adsorbent when pressure is 0 2 atm 1 0 4 2 0 6 4 0 2 3 0 8 m