Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Chemical kineticsConsider the reaction KCIO3 6 FeSO4 3H SO4 KCI 3Fe SO4 3 3H O If concentration of FeSO decreases from 0 9 M to 0 3 M in 10 second the rate of formation of KCI is in the above reaction would be In M sec

Physical Chemistry
General156 Not considering the electron spin the degeneracy of second excited state of H atom is 9 while the degeneracy of the first excited state of H is a 1 b 2 c 3 d 4

Physical Chemistry
Electrochemistry2 5 Faraday of electricity is passed through solution of CuSO The number of gram equivalents of copper deposited on the cathode would be a 1 4 b 2 e snad c 2 5 lousalsa d d 1 25

Physical Chemistry
Chemical kinetics9 In the reversible reaction 2NO N O4 K rate of disappearance of NO is equal to 2 K 1 2K NO 1 K 27 2K NO 1 2K N O4 3 2K NO 1 K NO

Physical Chemistry
Gaseous and liquid states20 mL of methane is completely burnt using 50 mL of oxygen The volume of the gas left after cooling to room temperature is 1 80 mL 2 40 mL 3 60 mL 4 30 mL

Physical Chemistry
SolutionsIn cold climate water freezes causing damage to the car radiator Ethylene glycol is used as antifreeze agent Calculate the amount of ethylene glycol to be added to 4 kg of water to prev it from freezing at 8 C K H O 1 86 K kg mot

Physical Chemistry
Chemical kineticsSecond order reactions Hydrolysis of ester by alkali Saponification CH COOC Hs NaOH CH COONa C H OH H 1 2HI 2HI H 1 2NO 2NO F NO 03 2CI O F 2NO O 2NO Fisio NO O 2Cl O For second order n 2 kt 1 1 1 2 1 a x a 1 a x 1 a

Physical Chemistry
Chemical kinetics43 In following compressibility factor v s pressure graph which is true 8 N Z B C pressure Z 1 10 9 1 At high pressure gas B behaves as non ideal 2 At low pressure gas B behaves as ideal gas olioj pat to nomy 3 At low pressure gas A behaves as ideal gas 4 At low pressure gas C behaves as ideal gas

Physical Chemistry
GeneralHow much amount of CuSO4 5H O required fo liberation of 2 54 g I when titrated with KI 2 4 99 gm 2 1 2 5 gm 3 2 4 gm 4 1 2 om to give

Physical Chemistry
General5 Dichromate ion in acid solution oxidizes stannous ion as 3Sn 14H Cr 0 3Sn 4 2Cr 7H C a If SnCl is the source of Sn2 how many g o SnCl would be contained in 2 litre of 0 1 M solution b If K Cr O7 is the source of Cr 07 what is the normality of solution containing 4 9g K Cr 0 in 0 1 litre of solution FITT 1005

Physical Chemistry
Atomic StructureFor an atom with atomic number 14 the number of orbits and orbitals in which electrons are present are respectively a 3 6 c 7 3 b 6 3 d 3 8

Physical Chemistry
EnergeticsCalculate the maximum temperature for the following reaction carried out under adiabatic conditon at constant pressure Assume O g is present in stoichiometric amount Given CH g 20 g CO g 2H O 4H 890 kJ mol AHO vap H O 45 kJ mol CPM CH g 35 J k mol CPM CO g 36 J k mol CPM H O g 32 J k mol all data provided at 298 K B 7702 K C 8298 K A 308 K D None of these

Physical Chemistry
General2 A gas can be liquefied most suitably at a T T and P P c T T and P P b T T and P Pc d T T and P Pe

Physical Chemistry
Atomic Structure10 155 Not considering the electron spin the degeneracy of first excited state n 2 of H atom is a 2 b 3 c 4 d 8

Physical Chemistry
General7 The reaction of white phosphorus with aqueous NaOH gives phosphine along with another phosphorus containing compound The reaction type the oxidation states of phosphorus in phosphine and the other products are respectively a redox reaction 3 and 5 b redox reaction 3 and 5 c disproportionation reaction 3 and 1 d disproportionation reaction 3 and 3

Physical Chemistry
General57 Amount of oxygen in 32 2g of Na2SO4 10H O is a 20 8 g c 2 24 g b 26 71 g d 2 08 g

Physical Chemistry
Solutions50 50 mL of 1 M oxalic acid is shaken with 0 5 g wood charcoal The final concentration of the solution after adsorption is 0 5 M What is the amount of oxalic acid adsorbed per gram of carbon b a 3 15 g c 6 30 g b 3 45 g d none of these vbmil Se

Physical Chemistry
Gaseous and liquid statesProblem 10 2 At 300 K a certain mass of a gas ocupies 1 104 dm volume Calculate its volume at 450 K and at the same pressure

Physical Chemistry
Generalof another Pressure of 1 g of an ideal gas A at 27 C is found to be 2 bar When 2 ideal gas B is introduced in the same flask at same temperature the pressure becomes 3 bar Find a relationship between their molecular masses

Physical Chemistry
SolutionsArtificial rain is caused by spraying a opposite charged collidal dust over a cloud b same charged collidal dust over a cloud c both d none of these

Physical Chemistry
ElectrochemistryCalculate the equilibrium 4Br O 4H EO 0 16 V cell constant for the cell reaction 2Br 2H O

Physical Chemistry
Energeticsd C In C In T T 4TT 2 Wildcom 70 Assuming ideal gas behaviour the AS for the isothermal mixing of 0 8 mole N and 0 2 mole of O is In 2 0 7 In 10 2 3 a 0 96 cal K b 0 32 cal K c 0 96 cal K d 0 32 cal K 6 If all the following gases are in monoatomic form which has great

Physical Chemistry
General15 The normal boiling point of a liquid X is 400 K Which of the following statement is true about the process X 1 X g a at 400 K and 1 atm pressure AG 0 b at 400 K and 2 atm pressure AG ve c at 400 K and 0 1 atm pressure AG ve d at 410 K and 1 atm pressure AG ve

Physical Chemistry
Chemical kinetics70 For the reaction 3A 2B C Products the following kinetic data was obtained Initial concentration A mol L B mol L C mol L 0 01 0 02 0 01 0 01 0 005 0 005 0 010 0 005 0 01 0 01 0 01 0 02 Initial rate mol L s 1 2 Rate k A B C 3 Rate k A B C 4 Rate k A B C 5 x 10 5 5 x 10 5 2 5 10 5 20 10 5 Hence the rate of reaction is given by 1 Rate k A B C

Physical Chemistry
Chemical kinetics164 The enthalpies of formation of CO g and CO g at 298 K are in the ratio 2 56 1 For the reaction CO g C s 2CO g AH 177 5 kJ AH of CO g is 1 316 96 kJ mol 3 141 6 kJ mol 1 165 Which of the following properties has incorrect order 2 113 78 kJ mol 4 180 6 kJ mol

Physical Chemistry
Surface chemistry7 Which of the following will have the highest coagulating power for Fe OH 3 colloid 3 2 b SO2 4 c Ca d Al3 Artificial rain is caused by spraying a PO

Physical Chemistry
GeneralThe isotopic abundance of C 12 and C 14 is 98 and 2 respectively What would be the number of atoms of C 14 isotope in 12 g carbon sample 1 1 2 x 10 2 3 5 88 x 1023 2 3 01 x 1023 4 6 02 x 1023

Physical Chemistry
General5 If for some reason the parietal cells of the gut epithelium become partially non functional what is likely to happen a The pancreatic enzymes and specially the trypsin and lipase will not work efficiently b The pH of stomach will fall abruptly c Steapsin will be more effective d Proteins will not be adequately hydrolysed by pepsin into proteoses and peptones

Physical Chemistry
Generalrespectively 4 The pressure in bulb dropped from 2000 to 1500 mm of Hg in 47 minute when the contained O leaked through a small hole The bulb was then completely evacuated A mixture of O and another gas of mol wt 79 in the molar ratio 1 1 at a total pressure of 4000 mm of Hg was introduced Find the mole ratio of two gases remaining in the bulb after a period of 74 minute

Physical Chemistry
Atomic Structure4 Fluorine is stronger oxidizing agent than chlorine in aqueous solution This can be attributed to the property A heat of dissociation B electron affinity D heat of hydration C ionization potential Election offinity of the elements or ions is correctly shown in

Physical Chemistry
General20 The equilibrium constants for the reaction A 2 A at 500 K and 700 K are 1 10 0 and 1 x 10 5 The given reaction is CBSE AIPMT 1996 ron a exothermic c endothermic If is the b slow d fast fraction of HI dissociated

Physical Chemistry
Gaseous and liquid states13 A real gas obey Berthelot equation of gas that is pola P 1 The Boyle s temperature for this is a and b are constant Tower TV Vm b RT Rb 2a 3 Rb 2 4 a VRb 2a VRb

Physical Chemistry
Atomic StructureWhen the value of azimuthal quantum number is 3 the maximum and minimum values of spin multiplicity are a 1 8 c 6 1 b 8 1 d 7 0

Physical Chemistry
Gaseous and liquid states5 An ideal gas is is collected by downward displacement of according to water Selcet the correct expression for P the diagram dHg 13 6 g cm gas Gas 1 P gas 2 P gas h cm H O d 1 g cm Patm aq Tension h 13 6 Patm hdg 3 P Patmaq Tension h 13 6 gas 4 None of these

Physical Chemistry
Solid stateA C They melt over a range of temperature There is no orderly arrangement of particles B D They They are rigid and incom The number of hexagonal faces that are present in a truncated octahedron is 2 B 4 C 6 D 8 A The number of atoms contained in a fcc unit cell of a monoatomic substance is D 6

Physical Chemistry
General17 K of a CaSO4 5H O is 9 x 106 Find the volume sp of CaSO for 1gm Mw 136 4 1 2 45 litre 3 4 52 litre 2 5 1 litre 4 3 2 litre

Physical Chemistry
EquilibriumThe preparation of SO g by raction SO g O g SO g 20 0 is an exothermic reaction If the preparation follows the following temperature pressure relationship for its yield then for temperatures T T T3 The correct option is 1 T3 T T 2 T T T3 3 T T T3 2 4 Nothing could be predicted about temperature through on yield 50 40 30 20 10 T T T 1 2 3 4 Pressure atm

Physical Chemistry
GeneralAn experiment requires minimum activi produced at the rate of 346 B particles per minut The half life period of 22 Mo which is a B emitter 66 6 hr Find the minimum amount of M required to carry out the experiment in 6 909 hr

Physical Chemistry
Solutions0 7 g of Na CO xH O is dissolved in 100 ml 20 ml of which required 19 8 ml of 0 1 NHCI The value of x is 1 4 2 3 3 2 4 1 diluted bu

Physical Chemistry
SolutionsThe number of unit cells in a cube shaped ideal crystal of NaCl is 512 The number of unit cell along each edge of the ideal crystal is

Physical Chemistry
Solid stateIf radius of an octahedral void is r and atomi radius of atoms assuming cubical close packing R Then the relation between r and R is 1 r 2R 2 r 1 414 R 3 r 0 414 R R 4 r

Physical Chemistry
GeneralMole fraction of ethanol in ethanol water mixture is 0 25 Hence percentage concentration of ethanol by weight of mixture is 1 25 2 75 3 46 4 54
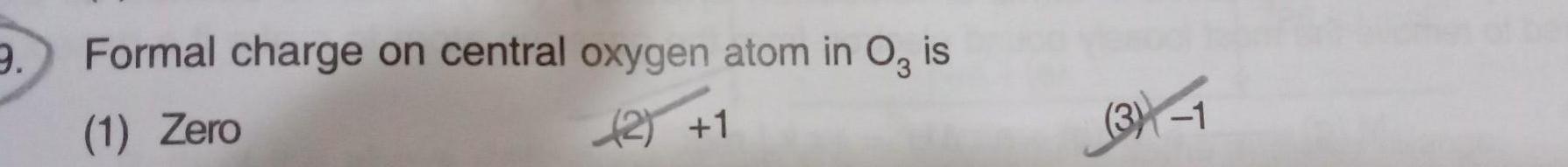

Physical Chemistry
General2 200 mL of a solution of mixture of NaOH and Na2CO3 was first titrated with phenolphthalein and N 10 HCl 17 5 mL of HCl was required for the end point After this methyl orange was added and 2 5 mL of same HCI was again required for next end point Find out amounts of NaOH and Na CO3 in mixture

Physical Chemistry
Chemical kineticsWhich of the following does not affect the rate of reaction 1 Amount of the reactant taken 2 Physical state of the reactant 3 AH of reaction 4 Size of vessel

Physical Chemistry
Energetics39 The AH N O5 g in kJ mol on the bases of the following data is AH 114 kJ mol AH 102 6 kJ mol 1 15 1 2NO g O g 2NO g 4NO g O g 2N O5 g A H NO g 90 2 kJ mol 2 30 2 3 36 2 4 none of these

Physical Chemistry
Chemical kineticsThe fraction of total volume occupied by the cube is 4 382 382 Simple cube is 36 atoms present in a simple 1 R 6 2 TU 3 2 3 TU 4 2 4 4

Physical Chemistry
General59 A manometer is connected to a gas containing bulb The open arm reads 43 7 cm where as the arm connected to the bulb reads 15 6 cm If the barometric pressure is 743 mm mercury What is the pressure of gas in bar

Physical Chemistry
Generald Mole fraction In a compound C H N atoms are present in 9 1 3 5 by weight Molecular weight of compound is 108 Its molecular formula is AIEEE 2002 a C H6N2 c C6H8N2 b C3H4N d C H12N 3

Physical Chemistry
Atomic StructureIf an electron can have three values of spin quantum numbers 0 and which of 1 1 2 2 the following statement would be correct a Li would have in the first period of the periodic table b The total number of elements is the second period would have been ten c There would have been 27 elements in the fourth period of periodic table d Number of periods would have been less than the in modern periodic tabl