Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
GeneralCalculate wt of urea which must be dissolved in 400 g of water so final solutions has V P 2 less than V P of pure water Let V P be V of water P P 02 V

Physical Chemistry
Chemical kineticsWhich of the following is are correct for a first order reaction The extent of reaction is equal to 1 e Concentration of the reactant decreases exponentially with time Concentration of the product increases exponentially with time A plot of logarithm of concentration of reactant versus time is linear with negative slone A B C D
Physical Chemistry
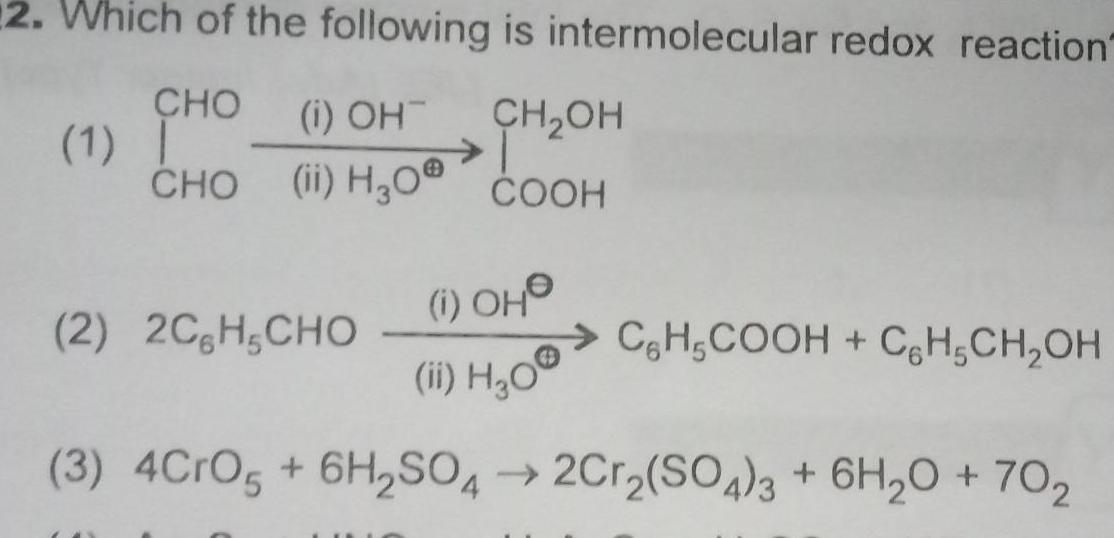
General2 Which of the following is intermolecular redox reaction CHO i OH CH OH CHO ii HgO COOH 1 1 OH ii H O 3 4CrO5 6H SO4 2Cr SO4 3 6H O 702 2 2C H CHO CH5COOH CH CH OH

Physical Chemistry
Atomic Structure74 Among the triatomic molecules ions BeCl N3 N O NO2 O3 SCI ICl2 I3 and XeF the total number of linear molecules s ion s where the hybridization of the central atoms does not have contribution from the d orbital s is JEE Adv 2015 Atomic number S 16 Cl 17 I 53 and Xe 54

Physical Chemistry
EquilibriumAp Ke following reaction 1 PCI 2 2SO O 3 N 3H 2NH 2 4 2 and 3 both log log RT 0 is true relationship for the PCl Cl 2SO

Physical Chemistry
General4 9 47 m 7100 gm of a mixture of CaCO3 and Na CO was ignited strongly which forms 11 2 L of CO at STP Then the mass ratio of Na CO3 and Caco in the mixture will be 1 1 4 3 2 1 2 4 1 4 1 1 llowing has maximum number o

Physical Chemistry
General29 28 30 31 32 1 33 B 1 litre Which of the following has the highest mass A 1 g atom of C B mole of CH4 10 2 D 3 01 x 1016 condensed to water occupies an approximate volume of C 1 ml D 18 ml C 10 ml of water A person adds 1 71 gram of sugar C12H22011 in order to sweeten his tea The number of carbon atoms added D 3 011 1023 atoms of oxygen are mol mass of sugar 342 A 3 6 1022 B 7 2 x 1021 C 0 05 D 6 6 10 2 500 ml of a gaseous hydrocarbon when burnt in excess of O gave 2 5 It of CO and 3 0 It of water vapours under same conditions Molecular formula of the hydrocarbon is A C4H8 B C4H10 C C5H10 D C5H12 On analysis a certain compound was found to contain iodine and oxygen in the ratio of 254 80 The formula of the compound is At mass I 127 O 16 A 10 B 1 0 C 1502 D 1 05 A compound contains 38 8 C 16 0 H and 45 2 N The empirical formula of the compound would be A CH3NH C C H5CN D CH2 NH 2 B CH3CN A giant molecule contains 0 25 of a metal whose atomic weight is 59 Its molecule contains one atom of that metal Its minimum molecular weight is A 5900 B 23600 C 11800 D 100 59 0 4 Element A reacts with oxygen to form a compound A O3 If 0 359 gram of A react to give 0 559 gram of the tomic weight of A will be D 47 9

Physical Chemistry
Chemical kinetics14 Consider the plot given 1 2 NH4NO aq N g 2H O l The rate law expression for the reaction can be written as k rate constant 3 4 ON HN Time t d NH NO k dt d NH4NO K NH NO dt K NH NO 2 d NH NO K NH NO 1 4 d NH NO dt

Physical Chemistry
Gaseous and liquid states998 2 One way of writing the equation of state for real gases where B is a constant is PV RT 1 B V 3 Derive an approximate expression for B in terms of van der Waals constants a and b IIT May 19971

Physical Chemistry
Surface chemistry147 Which of the following molecules is most suitable to disperse Daba benzene in water a Nexsus or c BMJ 3129 b Nat O ods 25midreuo O Nat O sortuod rdslieve zi cis aydate di a 6011 lor O Nat c CH3 AV

Physical Chemistry
Solutionsive 6 66 ml of a solution containing HCI with KIO is treated with excess of KI and I liberated and it titrated with 400 ml 0 1 M hypo solution Calculate molarity of KIO in solution

Physical Chemistry
General9 A mixture of N and H is caused to react in a closed container to form NH3 The reaction ceases before any of the reactant has been totally consumed At this stage 4 moles each of N H and NH3 are present Then the weight of N and H present originally were respectively 1 224 g and 16 g 3 168 g and 16 g 2 168 g and 20 g 4 224 g and 20 g

Physical Chemistry
Surface chemistry68 Which gas would get adsorbed when passed into a solution of A1 aq a NH b NO c CO stafton 4 d 0

Physical Chemistry
Solutionsx mole of KCI and y mole of BaCl are both dissolved in 1 kg of water Given that x y 0 1 and K for water is 1 86 K molal What is the observed range of AT if the ratio of x and yi varied 1 0 37 to 0 55 3 0 56 to 0 93 2 0 185 to 0 93 4 0 37 to 0 93

Physical Chemistry
Electrochemistry3 Which among the following has maximum potential for the half cell reaction 2H 2e H A 1 0 M HCI B 1 0 M NaOH C Pure water D A solution with pH 4

Physical Chemistry
Chemical Bondingf we have 10 molal urea solution Calculate mole fraction of urea in this solution also calculate o w w of urea MW 60 O moles urea in 1000 g of water

Physical Chemistry
Equilibrium2 The hydrogen ion concentration of a 10 8 M HCI aqueous solution at 298 K K 10 14 is W AIPMT Prelims 2006 2 1 0525 10 7 M 4 1 0 10 8 M 1 1 0 x 10 6 M 3 9 525 x 10 8 M

Physical Chemistry
Solutions2 The mole fraction of water in 20 wt wt aquec solution of H O2 is 68 77 a 77 68 b c 20 d 80 80 20

Physical Chemistry
GeneralOxidation of succinate ion produces ethylene and carbon dioxide gases On passing 0 2 Faraday electricity through on aqueous solution of potassium succinate the total volume of gases at both cathode and anode at STP 1 atm and 273 K is JEE MAINS ONLINE 2016 1 8 96 L 2 2 24 L 3 4 48 L 4 6 72 L

Physical Chemistry
GeneralKTRA CREDIT It was found that 16 g SO were formed from the reaction of iron IV sulfide and

Physical Chemistry
GeneralCaCO3 decomposes to give CaO and CO if the masses of CaO and CO produced are 5 6 g and 4 4 g respectively by heating 12 g of an impure CaCO3 sample then the impurity of the sample will be 1 33 33 2 16 67 3 83 33

Physical Chemistry
General1 2 g mixture of two divalent metals A at wt 30 and B at wt 15 on reacting with dilute HCI of dide KEY o tosolution gives 2 24 L H gas at NTP then 2 composition of A in g 1 1 2 0 5 La 3 1 5 08 4 1 2 22 2 8

Physical Chemistry
GeneralA mixture of potassium chlorate oxalic acid and sulphuric acid is heated During the reaction which element undergoes maximum change in the oxidation number a S c Cl b H d C Prelims 2012

Physical Chemistry
GeneralYellow light emitted from a sodium lamp has a wavelength A of 580 nm Calculate the frequency v wave number and energy of yellow light photon

Physical Chemistry
Nuclear chemistryThe volume of the blood in a patient is estimated by recording the activity of Na 4 administered into the patient s blood Find the volume in of the blood if the activity after 25 hrs is 8 dpm me Given the initial activity of Na24 sample is 2 10 Bq when administered the t for Na 4 15 hrs use 32 3 3 1 2

Physical Chemistry
General102 NaOH then nature of resulting solution and 0 6 N CAN normality of excess of reactant left is D 0501 M 10 1 Acidic N 5 N blom 2 Basic M N 5 mi of N

Physical Chemistry
Generalc 500 500 A sample of an alloy of silver weighing 0 50 g and containing 90 silver was dissolved in conc HNO3 and silver was analysed by Volhard method A volume of 25 ml of a KCNS solution was required for complete precipitation The normality of KCNS solution is Ag 108 a 4 167 c 3 136 b 0 167 d 0 125

Physical Chemistry
General19 For the reaction 2NO g 2NO g O g K 1 8x10 6 at 185 C The value of K for the reaction NO g NO g O g at the same temperature is S a 1 34 10 b 1 8 10 6 09x 10 3 d 1 8 106

Physical Chemistry
Equilibrium123 Buffering action of a mixture of CH COOH and CH COONa is maximum when the ratio of salt to acid is equal to 1 1 0 3 10 0 2 100 0 4 0 1

Physical Chemistry
GeneralThe most stable complex among the following is 2 Co NH3 2 4 Mn NH3 2 1 Fe NH3 612 3 Ni NH3

Physical Chemistry
Electrochemistry14 2 V 4 0 12 V 13 The equilibrium constant for the reaction 2MnO 6H 5H C O42Mn 8H O 10CC is approximately Given that E 2 MnO Mn 1 51 V A 00 0 0 49 V 1 10295 2 10339 3 10172 4 10490 On dilution specific conductance

Physical Chemistry
SolutionsSOIS are also called reversible colloids because a they can be reformed by mixing residue dispersed phase in dispersion medium even after drying b they can be easily precipitated from the colloidal system c once formed the dispersion medium and dispersed phase cannot be separated d special reversible reactions are used to prepare

Physical Chemistry
GeneralWhen an ideal gas undergoes unrestricted expansic no cooling takes place because the molecules a Exert no attractive forces on each other b Do work equal to loss of KE c Collide without loss of energy d Are above the inversion temperature

Physical Chemistry
EquilibriumIf the forward rate constant of reversible reaction is 0 16 and backward rate constant is 4 104 then the equilibrium constant will be 1 2 25 x 10 6 2 2 5 10 5 3 4 x 10 6 4 4 x 10 5

Physical Chemistry
General5 Tyndall effect is more pronounced in a hydrophilic sols c lyophilic sols b hydrophobic sols d Both a and c

Physical Chemistry
GeneralAn equilibrium mixture at 700 K of 0 50 M N 3 00 M H and 2 00 M NH is present in a container Now if this equilibrium is disturbed by adding N so that its concentration becomes 1 50 M just after addition then which of the following graphs represents the above situation more appropriately Initial og Now oq Initial og Now oq A 2 0 1 0 C 3 0 2 0 1 0 Time Initial eq New eq Time NH NH B D 1 conc Conc M 2 0 1 0 3 0 2 0 1 0 Time Intial og New oq NH H N NH H N Timo 050 M N 3 00 MH 2 00 M NH fer

Physical Chemistry
Equilibrium31 The unit of equilibrium constant Ke for the reaction A B C would be a mol L c mol L b mol L I d no unit

Physical Chemistry
GeneralWhich of the following changes with increase in temperature AIEEE 2002 a Molality b Weight fraction of solute c Fraction of solute present in water d Mole fraction

Physical Chemistry
SolutionsThe ratio of the value of colligative properties K Fe CN aq to that of Fe Fe CN 3 aq is Assuming 100 dissociation 1 4 3 3 5 7 2 3 4 4 7 5

Physical Chemistry
Generaln moles of an ideal triatomic linear gas undergoes a process in which the temperature changes with volum as T k V where k is a constant Choose incorrect alternative 5 A At normal temperature C R BAt any temperature C C R C At normal temperature molar heat capacity C 3R DAt any temperature molar heat capacity C 3R

Physical Chemistry
Surface chemistryOne desires to prepare a positively charged sol of silver iodide This can be achieved by AC Ag ingen a adding a little AgNO solution to KI solution in slight 3 shi 3Tuners an 3 excess b adding a little KI solution to AgNO solution in slight ved cemper 201 3 10 07 excess 5265 10 NOT and KI ne fol a artis outdo d none of these c mixing equal volumes of equimolar solutions of AgNO I on AgNo 1 AgI KNO 9 203 o ghar pogl

Physical Chemistry
Solutionscapacity 500 One of these beakers labeled as A was filled with 400 mL water whereas the beaker labeled B was filled with 400 mL of 2 M solution of NaCl At the same temperature both the beakers were placed in closed containers of same material and same capacity as shown in figure B C A D water At a given temperature which of the following statement is correct about the vapour pressure of pure water and that of NaCl solution A Vapour pressure in container A is more than that in container B B NaCl solution Vapour pressure in container A is less than that in container B Vapour pressure is equal in both the containers Vapour pressure in container B is twice

Physical Chemistry
Chemical kineticsWhich of the following value of AH represent that the product is least stable 1 94 0 k cal mol 2 231 6 kcal mol 3 21 4 kcal mol 4 64 8 k cal mol For the auto ionization of water at 27 C H OH aq OH aq equilibrium constant is 10

Physical Chemistry
Generalis due to adsorption of a H b S c OH 72 At CMC the surfactant molecules a decompose c associate 3 d 0 74 d b become completely soluble d dissociate and 5 itsad Arsenic sulphide in mering sol The reagent with least is negative

Physical Chemistry
GeneralGiven X Na HASO Y NaBrO ZHCl NaBr H AsO NaCl The values of X Y and Z in the above redox reaction are respectively 2 3 1 6 1 2 1 3 3 2 1 2 JEE Main o 4 3 1 4
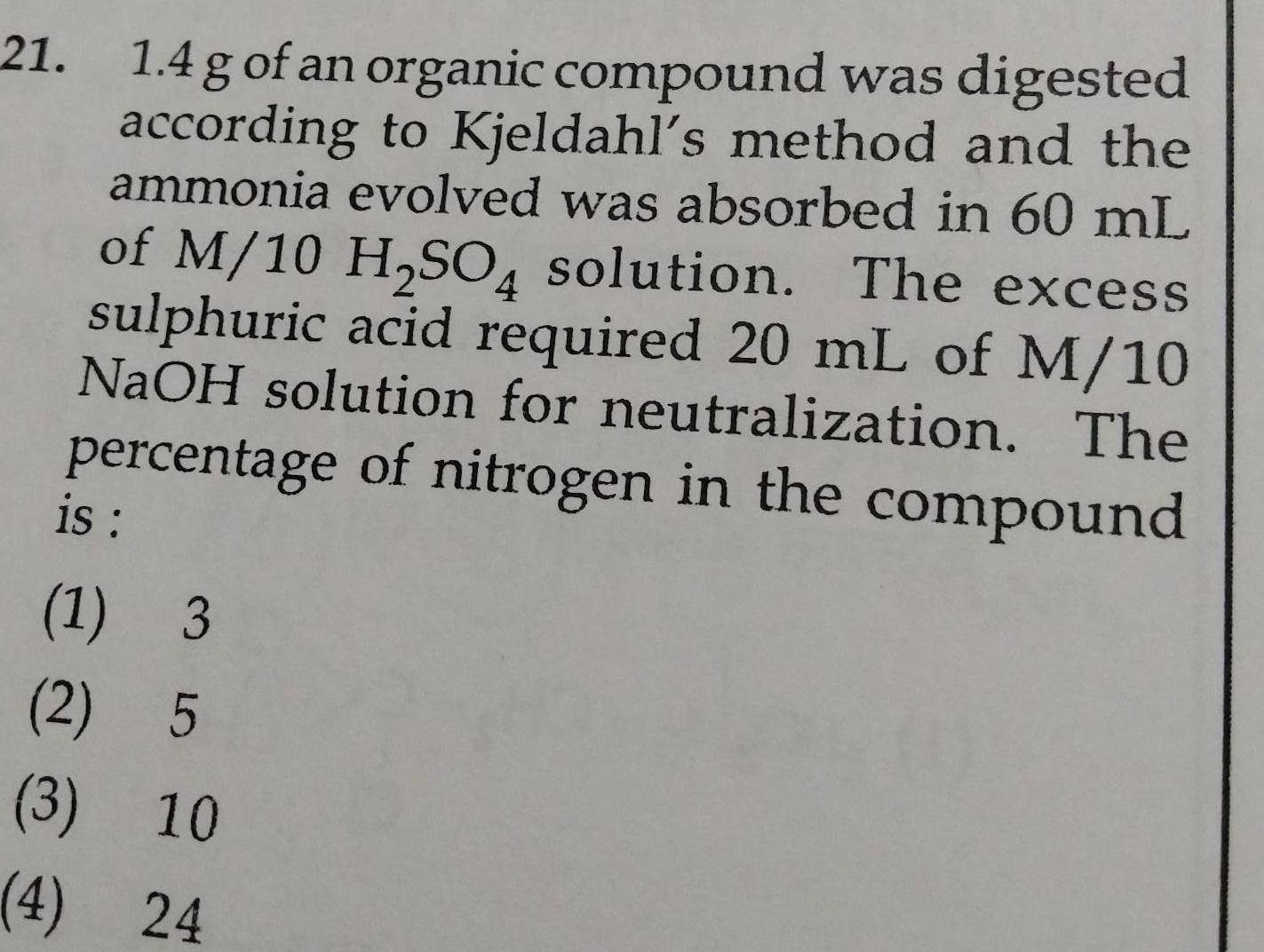
Physical Chemistry
General21 1 4 g of an organic compound was digested according to Kjeldahl s method and the ammonia evolved was absorbed in 60 mL of M 10 H SO4 solution The excess sulphuric acid required 20 mL of M 10 NaOH solution for neutralization The percentage of nitrogen in the compound is 1 3 2 5 3 10 4 24

Physical Chemistry
Surface chemistryOn adding AgNO3 solution into KI solution a negatively charged colloidal sol is obta when they are in a 50 mL of 0 1 M AgNO3 50 mL of 0 1 M KI b 50 mL of 0 1 M AgNO3 50 mL of 0 2 M KI c 50 mL of 0 2 M AgNO3 50 mL of 0 1 M KI d None of these 1000 of AgNO solution in KI solution The charge likely to

Physical Chemistry
ElectrochemistryThe electrolysis of cold sodium chloride solution produces NaOH and Cl The Cl produced the disproportionately into NaOH solution to give sodium hypochlorite NaCIO and sodium chloride How long will a cell operate to produce 1 00 10 L of 7 45 w w solution of NaCIO if the cell current is 9 65 ampere Assume that the density of solution is 1 00 gm ml Fill your answer by multiplying it with 107 2

Physical Chemistry
Surface chemistryOn adding few drops of dil HCl to freshly precipitated ferric hydroxide a red colored colloidal solution is obtained This phenomenon is known as many box a peptisation b dialysis c protective action d dissolution 34

Physical Chemistry
EquilibriumTwo substances A and B are present such that A 4 B and half life of A is 5 minute and that of B is 15 minute If they start decaying at the same time following first order kinetics how much time will the concentration of both of them would be the same 1 15 minute 2 10 minute