Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Equilibrium9 K and K are equilibrium constant for reactions i and ii N2 g O2 g NO g a K K c K 1 7 2 2NO g N2 g 0 0 O2 g b K K d K K i ii 1989

Physical Chemistry
EnergeticsThe Enthalpy of formation of H SO4 at 298K will be Given S O SO SO2 1 2 O2 SO3 SO3 H O H SO4 H 1 2 O2 H O O 810 KJ O 710 KJ O 680 KJ O 510 KJ AH 300 KJ AH 100 KJ AH 130 KJ AH 280 KJ

Physical Chemistry
General10 AH of the reaction is equal to 13 C4H10 g O2 g 4CO2 g 5H O 1 2 NCERT Pg 176 1 AfH of CO2 2 AfH of H O 3 AfH of C4H10 4 AcH of C4H10 11 In isothermal free expansion of an ideal gas NCERT Pg 166

Physical Chemistry
Surface chemistrydeduced using the assumption CBSE PMT 2007 The adsorption takes place in multilayers The adsorption sites are equivalent in their ability to adsorb the particle The heat of adsorption varies with coverage The adsorbed molecules interact with each

Physical Chemistry
Solid state48 49 50 51 1 each face diagonal In face centred cubic lattice tetrahedral voids are present at Question Type Single Correct Type 2 each cube diagonal 3 body centre 52 53 54 55 56 4 each edge centre English Review

Physical Chemistry
ElectrochemistryThe molar conductance of NaCl HCl and CH3COONa at infinite dilution are 126 45 426 16 and 91 0 S cm mol 1 respectively The molar conductance of CH3CQOH at infinite dilution is Choose the right option for your answer 390 71 S cm mol 1 698 28 S cm 2 mol 1 540 48 S cm mol 1 201 28 S cm2 mol 1 1 2 3 4

Physical Chemistry
Solutions100 mL of FeSO4 solution required 200 mL of 0 1 M acidified KMnO4 solution for complete reaction What is the molarity of the FeSO4 solution MnO Fe H Mn Fe H O Select an answer A B 0 5 M 1 M 1 5 M

Physical Chemistry
Chemical Bonding3 1 Pz 3 02pz The bond order of N is same as that of NCERT Pg 129 1 N 3 02 2 N2 4 0 3 m n 20 Minimu 1 02 3 Oz

Physical Chemistry
Equilibrium4 A H 0 and AS 0 3 If equilibrium constant is 2 atm at 300 K then the standard free energy change at 300 K and 1 atm pressure is NCERT Pg 187 1 1728 9 J mol 2 1728 9 J mol 3 309 J mol 1 4 309 J mol In which of the following entropy decreases NCERT Pg 183 1 CaCO3 s CaO s CO2 g 2 2H a H g 2 Entropy 3 Internal en 4 Enthalpy 13 10 mol of id isothermally f work done du 1 1 91 kJ 3 19 1 kJ 14 If ArH of A

Physical Chemistry
Energetics4 Enthalpy 13 10 mol of ideal gas expanded reversibly isothermally from 1 L to 10 L at 100 K The work done during the process is NCERT Pg 166 1 1 91 kJ 3 19 1 kJ 14 If ArH of A B B C and A D are x y and 7 kJ mol 1 respectively then of AMERA 2 0 95 kJ 4 38 2 kJ L 2 2 CO 6 IM 4

Physical Chemistry
Solid stateIn a cubic lattice of XYZ X atoms are present at all corners except one corner which is occupied by Y atoms Z atoms are present at face centres The formula of the compound is XYZ 3 X Y Z 24 X7Y24Z

Physical Chemistry
Equilibrium1 94 At 80 C distilled water has H3O concentration equal to 1 x 10 6 mole litre The value of K at this temperature will be a 1 10 2 c 1 x 10 6 b 1 10 15 d 1 10 1994 9 J 95 According to Le Chatelier s principle adding heat to a solid and liquid in equilibrium will reac a

Physical Chemistry
Energetics1 mole ideal gas undergoes change in state from 2 atm 5 litre 300K to Pf Vf Tf P If w 6 93 atm litre Q If w 5 atm litre 1 2 R If w 30 atm litre 3 Process is irrreversible isothermal at Pext 1 atm Process is isobaric at 2 atm pressure with V 20 litr Process is reversible isothermal with 1 atm

Physical Chemistry
GeneralCalculate the enthalpy of combustion of pentanol from the given information Enthalpy of formation of pentanol AH 354 kJ mole O2 g C H OH 5C s Enthalpy of combustion of carbon and hydrogen are 395 5 kJ mole and 285 8 kJ mole respectivel 6H 2 g

Physical Chemistry
EquilibriumJ Asp 1 Which is are correct statement s about the solubility of AgCl s Given Ksp AgCl 10 0 K Ag NH3 2 108 a Solubility of AgCl in pure water is 10 5 gm litre b Solubility of AgCl in 2 M KBr is 10 5 mol litre c Solubility of AgCl in 2 M AgNO3 is 5 10 M d Solubility of AgCl in 2M NH 3 is 0 166 M 1 toujor Istra a l Til M

Physical Chemistry
Energetics60 1 mole of an ideal monoatomic gas initially at 1 atm and 300 K experiences a process by which pressure is doubled The nature of the process is unspecified but AU 900 cal The final volume will be 1 Given R 0 08 atm lit mol K 2 Cal K mol J

Physical Chemistry
EnergeticsQ 57 3 5 g of a fuel with molecular weight 28 was burnt in a calorimeter and raised the temperature of 1 g water from 25 C to 67 3 C If all the heat generated was used in heating water the heat of combustion of fuel is k cal

Physical Chemistry
Atomic StructureRadius of first excited state of Be ion is NCERT Pg 48 1 13 22 pm 2 52 9 pm 3 105 8 pm 4 211 6 pm Energy required to excite the electron in a hydrogen atom from 2nd to 3rd orbit is NCERT Pg 48

Physical Chemistry
GeneralIn the sugar factory sugar is produced from sugarcane The process 5M involves the following steps not in order a The separated crystals are sent to a dryer and the dried sugar is packed b Lime is added to the juice in a mixer and the coagulated impurities are separated with a filter c The sugar crystals are separated in a basket centrifuge and the mother liquor filtrate is again sent to the evaporator for further concentration d The concentrated juice is sent to a crystallizer where the sugar crystals are formed e The clarified juice is sent to an evaporator for concentrating the juice f Extraction of juice with a roller crusher

Physical Chemistry
Solutions4 Out of the following liquid pair which solution follows the positive deviation from Raoult s law 1 Acetone Chloroform 2 Water Nitric acid 3 Water Hydrochloric acid 4 Benzene Methanol

Physical Chemistry
GeneraleV Consider an electronic state y of Het whose energy The ground state energy of hydrogen atom is 136 azimuthal quantum number and magnetic quantum number are 3 4 eV 2 and 0 respectively Which of the following statement s is are true for the state y JEE Adv 2019 Paper 2 A It is a 4d state ADM B The nuclear charge experienced by the electron in this state is less than 2e where e is the magnitude of the electronic charge C It has 2 angular nodes D It hoc 3 radial podes

Physical Chemistry
GeneralWhich of the following represents an oxidation only change Select an answer 2 A Cu aq 2e Cu g B Mg s Fe aq Mg aq Fe s C Cl aq 2e 2Cl aq D Zn s 2e Zn s

Physical Chemistry
ElectrochemistryConsider a 70 efficient hydrogen oxygen fuel cell working under standard conditions at 1 bar and 298 K Its cell reaction is H g 10 H O The work derived from the cell on the consumption of 1 0 x 10 3mol of H g is used to compress 1 00 mol of a monoatomic ideal gas in a thermally insulted containe What is the change in the temperature in K of the ideal gas The standard reduction potentials for the two half cells are given below g 4 H aq 4e 2H O P E 1 23 V 2H aq 2e H g E 0 00V se F 96500 C mol R 8 314 J mol K 1

Physical Chemistry
Chemical kinetics5 In an acidic indicator HIn has ionization constant is 10 8 The acid form of indicator is yellow and alkaline form is red Which is correct statement bo Given log2 0 3 log3 0 48 a The pH range of indicator is 7 to 9 b Change in pH is 0 96 when 75 yellow colour change to 75 red colour c This indicator is suitable for the titration of strong acid vs strong base d pH of indicator is 8 3 when ratio of acid form to alkaline form is 2

Physical Chemistry
Nuclear chemistry9 Energy required to excite the electron in a hydrogen atom from 2nd to 3rd orbit is 1 3 63 10 1 J 3 3 00 x 10 19 J NCERT Pg 48 2 2 18 10 18 J 4 5 45 10 19 J 0 de Broglie wavelength of 20 g ball moving with a velocity of 50 ms is NCERT Pg 50 1 6 626 x 10 7 m 1 2 6 626 x 10 34 m 1 m l L

Physical Chemistry
Generalthe estimation of nitrogen 1 4 g of an organic Aan compound was digested by Kjeldahl method and the evolved ammonia was absorbed in 60 mL of M 10 sulphuric acid The unreacted acid required 20 M mL of sodium hydroxide for complete 10 neutralization The percentage of nitrogen in the compound is JEE Main 2014 1 6 2 10 3 3 4 5

Physical Chemistry
GeneralA patient is required to received a 15 mg lb solution every 12 hrs The supply in the hospital is 1 2 g ml How many ml of solution does the patient need every 12 hrs Assuming that the patient is 50 kg Final answer should contain at least 4 SF

Physical Chemistry
GeneralL X gm of Ag was dissolved in HNO3 and the solution was treated with excess of NaCl When 2 87 gm of AgCl was precipitated The value of x is Ag 2HNO3 AgNO3 NO H O AgNO3 NaCl AgCl NaNO3 A 1 08 gm B 2 16 gm C 2 70 gm D 1 62 gm

Physical Chemistry
Atomic Structure13 Which of the following set of quantum numbers is not possible NCERT Pg 56 1 1 n 3 1 2 m 2 s 2 1 2 n 4 1 0 m 0 s NI 2 1 3 n 2 1 1 m 0 s 7 2 14 Total number of nodes subshell is 1 4 n 5 1 3 m 2 s 2 present in 3d INCER FOL 16 4 dxz Energy of which hydrogen atom 1 6s 3 4d 17 According to Aufba orbital takes pla multielectron atom 1 5p 3 4f 18 Maximum number

Physical Chemistry
Atomic Structure4 Energy of one mole of photons of radiation whose frequency is 2 x 1014 Hz is nearly 1 80 kJ 3 247 kJ NCERT Pg 43 2 153 kJ 4 366 kJ 2 3 4 h 5T SE 5h 2

Physical Chemistry
Atomic Structure16 Energy of which orbital is maximum for hydrogen atom NCERT Pg 61 1 6s 3 4d 2 5p 4 5f 17 According to Aufbau principle filling of which orbital takes place just after 5s in a multielectron atom NCERT Pq 621 20

Physical Chemistry
Solid stateA copper complex crystallising in a CCP lattice with a cell edge of 0 4518 nm has been revealed by employing X ray diffraction studies The density of a copper complex is found to be 7 62 g cm 3 The molar mass of copper complex is g mol 1 Nearest integer Given NA 6 022 x 102 mol en r

Physical Chemistry
ElectrochemistryMg2 Mg 0 80 V 0 8 Ag Ag 2 37 V Ecu Cu 0 34 V E EH H8 0 79 V Hg Which of the following statements is correct A AgNO aq can be stored in copper vesse B Cu NO aq can be stored in magnesium vessel C CuCl can be stored in silver vessel D HqC can be stored in copper yessel

Physical Chemistry
Chemical kinetics50 A gaseous reaction A g B g C g shows increases in pressure from 100 mm to 120 mm in 5 minutes The rate of disappearance of A is A 4 mm min 1 C 16 mm min 1 B 8 mm min 1 D 2 mm min 1
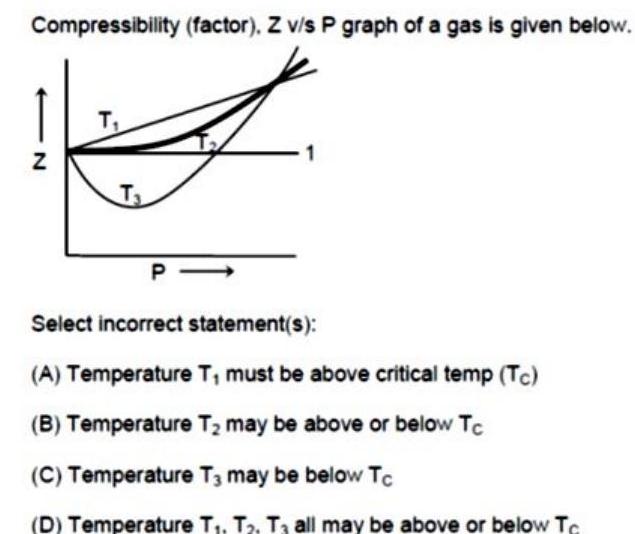
Physical Chemistry
EnergeticsCompressibility factor Z v s P graph of a gas is given below Z T T P Select incorrect statement s A Temperature T must be above critical temp Tc B Temperature T may be above or below Tc C Temperature T3 may be below Tc D Temperature T T T3 all may be above or below Tc

Physical Chemistry
Solid state2 54 An element A Atomic weight 100 having bcc structure has unit cell edge length 400 pm The number of atoms in 10 g of A is X 1022 unit cells

Physical Chemistry
ElectrochemistryMolar conductance of Al3 is 150 scm mole and equivalent conductance of SO is 200 scm eq at infinite dilution calculate equivalent conductance of Al SO4 1 225 2 300 3 250 4 290

Physical Chemistry
ElectrochemistryWhen Q amount of electricity passed through aq Cu NO3 6 35 gram of Cu deposited calculate the volume of H liberated when same Q electricity passed through electrolysis of acidified water Atomic weight of Cu 63 5 gr 1 11 2 lit 2 1 12 lit 3 2 4 lit 4 22 4 lit

Physical Chemistry
General1996 86 If a is dissociation constant then the total number of moles for the reaction 2HI H 1 will be a 1 c 2 b 1 a d 2 a 1996 87 The pH value of N 10 NaOH solution is b 13 a 12 c

Physical Chemistry
Gaseous and liquid statesPart A 10 moles of an ideal gas undergoes reversible isothermal expansion at 300 K from 5 L to a volume of 10 L 1 Will the entropy of gas increase decrease or remain same Calculate the entropy change in gas 2 Will any work be done by on the gas If yes then calculate the work done and explain meaning of sign of work done 3 Calculate the direction and magnitude of heat flow with the surroundings 4 Will the entropy of surroundings change too remember its a reversible isothermal process If yes then calculate the change 5 What will be the change in the entropy of universe Part B Considering the expansion in part A to be irreversible answer all the five parts asked in Part A

Physical Chemistry
Generalthe mole of an ideal gas with Cvm 3R 2 indipendently of the temperature which was initially at 300K and 1 atm is compressed adiabatically so that the final temperature is 350K Find q w the change in U and the change in H all in joules J for the process

Physical Chemistry
ElectrochemistryThe CORRECT option s regarding extraction of pure aluminium from red bauxite is are Serpeck s process is used for ore concentration comes as filbrate B Leaching of ore with aq NaOH produces precipitate of NaAlO C Weakly acidic medium is used to precipitate Al OH from solution containing Na Al OH ppt D Only pure Al 0 is used as electrolyte in Hall Heroult s process of electrolytic reduction ugh Work

Physical Chemistry
Solutions161 0 01 molal solution of Pt NH Cl in water had a freezing point depression of 0 054 C If K of water is 1 8 the correct formula for above molecule is A Pt NH3 4Cl CI B PI NH3 4Cl JCl C Pt NH3 4Cl CI D PI NH3 4 Cl4 A 168

Physical Chemistry
Gaseous and liquid states83 The solubility product of CuS Ag2S and H are 10 31 10 44 and 10 54 respectively T solubilities of these sulphides are in the orde a HgS Ag S CuS b CuS Ag2S HgS c Ag S CuS HgS d AgS HgS CuS 1997 84 The equilibrium constant for the reaction N N 3H NH ic K th

Physical Chemistry
Chemical BondingWhich among the following factors is the most important in making fluorine the strongest oxidizing halogen 2 Ionization enthalpy 4 Bond dissociation energy 1 Hydration enthalpy 3 Electron affinity

Physical Chemistry
GeneralSolveLancer Test Quicklime is soluble in water If we want to dissolve 3 g of quicklime in water then minimum amount of water required will be SolveLancer Test a 2g b 18g c 3g d 0 96g ERK 560

Physical Chemistry
General23 An organic compound C H 0 was bu the amount of oxygen needed for complete combustion to CO and H O The hot gases when cooled to 0 C and 1 atm pressure measured 2 24 litres The water collected 00110 010 during cooling weighed 0 9 g The vapour pressure of pure water at 20 C is 17 5 mm Hg and is lowered by 0 104 mm when 50 g of the organic compound are dissolved in 1000 g of water Give the molecular formula of the organic compound al or gason 1983

Physical Chemistry
Surface chemistrySolveLancer Test The temperature at which the solubility of surfactant is equal to surfactant s CMC SolveLancer Test a Kraft s temperature b Boyle s temperature c Critical temperature d None of these

Physical Chemistry
Generala Calculate the kinetic energy of a photoelectron emitted by a sodium surface when light of wavelength 400 nm is incident on it The work function of sodium is 2 28 eV b Calculate the value of the longest wavelength which can result in the emission of a photoelectron from a sodium surface

Physical Chemistry
GeneralWhen a gaseous sulphur dioxide and hydrogen sulphide mix in the presence of water the reaction SO2 2H S 2H O 3 S occurs Here hydrogen sulphide in acting as Select an answer A B C An oxidizing agent A reducing agent A dehydrating agent A catalyst