Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
ElectrochemistryA steady current of 1 5 amperes was passed through two electrolytic cells A and B containing CuSO4 and AgNO3 respectively and connected in series If 1 45 g of Ag is deposited at the cathode of cell B than the mass of copper deposited in cell A will be NCERT Pg 94 47 0 43 g 2 0 36 g 3 0 51 g

Physical Chemistry
Chemical kineticsFor the reaction 2A 3B product A is present in excess When concentration of B is changed from 0 03 M to 0 06 M rate is doubled Thus rate law is 1 dx K A B 2 dx K A B dt 3 dx K A B 4 dx K A B dt

Physical Chemistry
SolutionsAn azeotropic solution of two liquids has a point lower than either of the boiling points of th two liquids when it 1 Shows negative deviation 2 Shows positive deviation 3 Shows no deviation turated

Physical Chemistry
GeneralDetermine the total heat in Joules U required for 15 g of ice to change from a temperature of 20 C to 125 C Heat of Vaporization Melting point C 0 Boiling Point C 100 Temperature C Heat of Fusion 6 02 40 7 C solid 37 6 C liquid c 75 4 C vapor re 33 1

Physical Chemistry
Chemical kineticsIder the following B Ea Reaction 1 A Reaction 2 C If the initial temperature is 400 K for both reaction and the rate constants are equal then find the ratio of Arrhenius factor A A for reaction 1 and 2 O 12 18 2 71 132 ctions 28 31 kJ D E 20 kJ a

Physical Chemistry
GeneralChoose the incorrect statement a Vitamin B is also known as thiamine b Riboflavin is another name for vitamin B c Vitamin E is also called pyridoxine d Ascorbic acid is another name for Vitamin C

Physical Chemistry
EquilibriumNO3 NO2 acidic medium E 0 79V NO3 NH OH acidic medium E 0 731V At what pH the above two half reaction will have same E values Assume the concentration of all other species to be unity

Physical Chemistry
Atomic Structure15 If n l are principal and azimuthal quantum no respectively then the expression for calculating the total no of electron in any energy level is l n 1 2 2l 1 l 0 3 l n 1 2 2l 1 l 0 l n 1 2 2 2l 1 l 1 l n 1 4 2 2l 1 l 0

Physical Chemistry
GeneralHow many mL of 0 250 M KMnO4 are needed to react with 3 55 g of Iron II sulfate FeSO4 The reaction is as follows 10FeSO4 aq 2KMnO4 aq 8H SO4 aq 2MnSO4 aq K SO4 aq 5Fe SO4 3 aq 2H O 1

Physical Chemistry
SolutionsA 5 solution w W of cane sugar molar mass 342 g mol has freezing point 271 K What will be the freezing point of 5 glucose molar mass 18 g mol in water if freezing point of pure water is 273 15 K a 273 07 K c 273 15 K b 269 07 K d 260 09 K

Physical Chemistry
General4 1 8 mole lit 3 0 9 mole lit 4 1 8 mole lit 3 A certain zero order reaction has k 0 025 M s for the 48 f 0 025 M S disappearance of A What will be the concentration of A after 15 seconds if the initial concentration is 0 50 M 1 0 5 M 2 0 375 M A 1 0 5 M 2 0 3 3 0 125 M 4 0 06 M 3 0 125 M 4 0 In the reaction NH NO aq N 0 HO

Physical Chemistry
Chemical kinetics53 The reaction of NO g and O g is first order in NO g 53 and 0 9 2NO2 9 03 g N O5 g O2 g The reaction can take place by mechanism 1 NO 03 Slow NO3 0 NO3 NO N O5 II 0 fast 0 0 NO O 0 Slow NO NO fast N O Select correct mechanism 1 I only 2 II only 3 Both I and II 4 None fast NO

Physical Chemistry
GeneralSolveLancer Test Consider the reaction given below BaCl aq 2AgNO3 aq 2AgCl Ba NO3 2 aq 1 56 g of BaCl2 limiting reagent reacts to give 2 01 g of AgCl calculate the percentage yield of the reaction Given Molar mass of Ba and Ag is 137 g mol and 108 g mol respectively SolveLancer Test a 90 b 93 4 c 84 5 d 88

Physical Chemistry
GeneralSolveLancer Test During crystallization the filtrate that contains the impurities and very minute quantity of the compound is called as SolveLancer Test a Mother liquor b Impure filtrate c Crystallized liquid d Salt induced liquid

Physical Chemistry
ElectrochemistryThe half cell potential of a half cell A A Pt were found to be as follows Percent of reduced form 48 8 Cell potential N 0 115 24 4 0 101 Determine the approximate value of n 42 Hm xum

Physical Chemistry
GeneralThe manganate and permanganate ions are tetrahedral due to 1 The T bonding involves overlap of p orbitals of oxygen with d orbitals of manganese 2 There is no bonding 3 The T bonding involves overlap of p orbitals of oxygen with p orbitals of manganese 4 The T bonding involves overlap of d orbitals of oxygen with d orbitals of manganese

Physical Chemistry
GeneralWhich of the following sets of quantum numbers is not allowed SOOJU I show Donje DJ woodwa d Options 1 n 4 1 3 m 3 s 2 x n 2 1 1 m 0 s 4 3 n 1 1 0 m 0 s AP EAMCET 2020 AP EAMCET 2020

Physical Chemistry
EquilibriumSolution A consists of a 0 20 M aqueous solution of formic acid HCOOH at 25 C Calculate the pH of Solution A The pK of HCOOH is 3 75 Answer
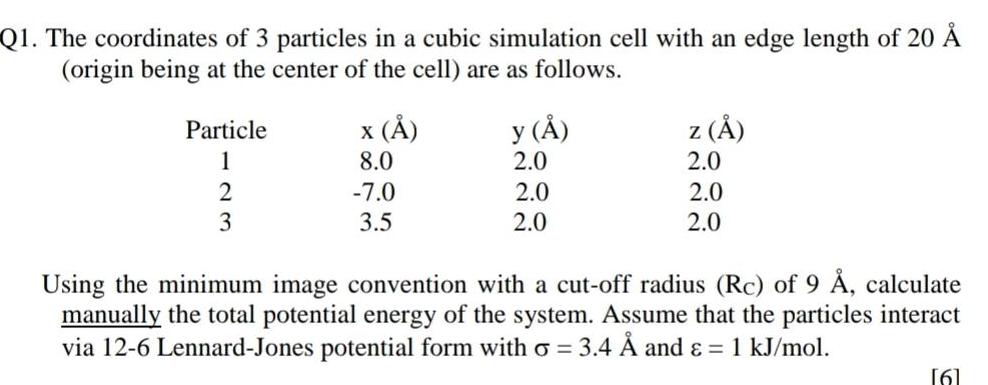
Physical Chemistry
Atomic StructureQ1 The coordinates of 3 particles in a cubic simulation cell with an edge length of 20 origin being at the center of the cell are as follows Particle 1 2 3 x 8 0 7 0 3 5 y 2 0 2 0 2 0 z 2 0 2 0 2 0 Using the minimum image convention with a cut off radius Rc of 9 calculate manually the total potential energy of the system Assume that the particles interact via 12 6 Lennard Jones potential form with o 3 4 and 1 kJ mol 6

Physical Chemistry
Equilibrium4 A substance undergoes a series of chemical reaction as shown A C KD with rate constant K B In 2 2000 C 200 sec K A K 20 In 2 sec What will be the value of once steady state is obtained C represents concentration A 40000 B 20000 D 400 In 2 10 sec Page No

Physical Chemistry
Chemical kineticsThe gas phase reaction 2NO g H g 2N OH g was carried out and the rate law was experimentally found out to be r K NO H Consider the two mechanism 1 2NO g N O fast 2N OH g slow NOH g fast 2N OH g slow Then the mechanism consistent with the observed rate law is N O g H g II NO g H g NOH g NO g Only I Only II Both I and II Neither I nor II

Physical Chemistry
Chemical kineticsFor the first order gaseous reaction X 2Yg Zg the initial pressure P 90 mm of Hg The pressure after 10 minutes is 180 mm of Hg The rate constant of the reaction is 1 15 10 3 sec 1 1 15x10 sec 1 2x10 sec 1 3

Physical Chemistry
Chemical kinetics27 For a first order homogeneous gaseous reaction A 2B C If the total pressure after time t was P and after long time t was P then k in terms of P P and tis 00 1 k 3 k 2 303 t 2 303 log P P P 2P log t 3 P P 2 k log 2 303 t 2 303 4 k log 2P P P P P P

Physical Chemistry
GeneralWe know that metal react with water to give metal oxide and hydrogen but in o ur textbook an equation is given like thi S 2k 2H O 2koH H Similarly sodium react with water to giv e sodium hydroxide Why is it not sodiu m oxide

Physical Chemistry
GeneralConsider the following statements about boric acid I It is a weak monobasic acid II It is not a protonic acid III It acts as a lewis acid The correct statement s among the following is are I only I and II only I and III only I II and III

Physical Chemistry
Solutions17 Equal moles of benzene and toluene are mixed the V P of benzene and toluene in pure state are 700 and 600 mm Hg respectively The mole fraction a benzene in vapour state is 1 0 7 3 0 50 2 0 47 4 0 54

Physical Chemistry
Gaseous and liquid states2 In the test tubes A and B shown here yeast was kept in sugar solution Which products of respiration would you expect in test tubes A and B i Acrobic Anaerobic Oil film F Sugar solution Yeast 7 Tes

Physical Chemistry
SolutionsTwo weak monobasic organic acids HA and HB have dissociation constants as 1 5 x 10 5 and 1 8 105 respectively at 25 C If 500 ml of 1 M solutions of each of these two acids are mixed to produce 1 litre of mixed solution what is the pH of the resulting solution

Physical Chemistry
General40 3733 E Sn HCI 1 O Conc HNO3 Conc H SO4 C 2 Br NaNO HCI 273 278K OH D Br 3 A Br Fe Boiling dil H SO4 NO E O Br B 4

Physical Chemistry
EquilibriumOxidation Reduction Reactions 1 Label the following half reactions as either an oxidation or reduction reaction a Br2 2e 2Br b Na Na e c 2C1 Cl 2e d Cl 2e 2C1 e Nate Na g Fe Fe 2e Cu 2e Cu h Fe e Fe BSESS

Physical Chemistry
Atomic StructureIf an element A shows two cationic states 2 and 3 and form oxides in such a way that ratio of the element showing 2 and 3 state is 1 3 in a compound Formula of the compound will be 1 Ag011 3 A 0 1 2 A 011 4 A5011

Physical Chemistry
General3 A 1 2 4 A 2 4 The rate constant of the reaction A 2B is 1 0 x 10 mol 47 fferal A afe A atr lit min If the initial concentration of A is 1 0 mole lit what would be the concentration of B after 100 minutes 1 0 1 mole lit B 2 0 2 mole lit 3 0 9 mole lit 4 1 8 mole li 1 0 1 mole 2 0 2 moles 3 0 9 mole

Physical Chemistry
Atomic Structurei 6 04 eV Match A Energy of ground state of He B Potential energy of I ii 27 2 eV orbit of H atom C Kinetic energy of II iii 8 72 x10 18 excited state of Het D Ionisation potential iv 54 4 eV of Het 1 A i B ii C iii D iv 2 A iv B iii C ii D i 3 A iv B ii C i D iii 4 A ii B iii C i D iv

Physical Chemistry
Chemical kineticsConsider a first order reaction at 450 K X g Y g Z g If value of rate constant K from given data is x Then value of 10 K is Assume initially only X is present Take In a 2 3 loga and log3 0 5 and log2 0 3 Time s 0 5 Total Pressure 15 20

Physical Chemistry
ElectrochemistryThe standard potential of the following cell is 0 23 V at 15 C and 0 21 V at 35 C Pt H g HCl aq AgCl s Ag s 2001 Write the cell reaction i Calculate AH and AS for the cell reaction by assuming that these quantities remain unchanged in the range 15 C to 35 C iii Calculate the solubility of AgCl in water at 25 C Given The standard reduction potential of the Ag aq Ag s couple is 0 80 V at 25 C

Physical Chemistry
EquilibriumWhich will make acidic buffer 50 mL of 0 1 M HCI 50 mL of 0 1 M NH3 50 mL of 0 1 M CH3COOH 50 mL of 0 1 M KOH 100 mL of 0 1 M HCI 50 mL of 0 1 M NaCl 100 mL of 0 1 M CH3COOH 50 mL of 0 1 M KOH

Physical Chemistry
Solid stateSolveLancer Test A compound was used as a solvent in dry cleaning of clothes The compound is a suspected carcinogen and pollutes ground water Which compound has replaced the compound these days SolveLancer Test 1 Liquefied carbon dioxide with suitable detergent 2 Liquefied carbon dioxide 3 Hydrogen peroxide a 1 2 b 2 3 c 3 1 d 1 2 3

Physical Chemistry
Atomic StructureTwo hydrogen atoms collide head on a nd end up with zero kinetic energy Eac h atom emits a photon of wavelength 1 21 6nm Which transition leads to this wavelength a 2 to 1 b 3 to 1 c 4 to 3 d 3 to 2 I looked up in the internet and there the y told that the lower orbit is n 1 since t he wavelength belonged to Lyman serie s But is it necessary that we should re member all the wavelength limits of ea ch series Can t we get both the orbit n

Physical Chemistry
Chemical kinetics5 Rate constant k 1 2 x 10 Ms and E 2 0 x 102 kJ 46 k mol When T 1 A 2 0 x 10 kJ mol 2 A 1 2 x 10 M s 3 A 1 2 x 10 M S 4 A 2 4 x 10 Ms T 1 A 2 0 x 2 A 1 2 x 3 A 1 2 x 4 A 2 4 x The rate constant of the reaction A 2B is 1 0 x 10 mol 47 aff

Physical Chemistry
Solid state8 A compound is formed by cation C and anion A The anions form hexagonal close packed hcp lattice and the cations occupy 75 of octahedral voids The formula of the compound is C A3 3 C A 2 C3A 4 CA

Physical Chemistry
Electrochemistry2 Consider the cell reaction H g Cl g 2H aq 2Cl aq Equilibrium constant for the cell at 298 K is given E C12 CI 1 20 volt and E H H2 0 volt 1 1040 7 2 1020 7 3 1030 7 4 1050 7

Physical Chemistry
Atomic StructureThe wavelength of first line of the Lyman series for hydrogen is 1216 A The wavelength for the first line of this series for a 10 time ionised sodiun atom z 11 will be 1 1000 A 3 10 A 2 100 A 4 1 A

Physical Chemistry
Chemical Bonding3 HNO 3HCl H Co 07 CM NO 3 Cill 4H 0 net carite the rate expression for this reaction 1 Calculate the value of kr giver HNO Reaction Reaction 0 8 1 C 07 Rote 0 0018 0 4 0 7 Reaction 0 4 Reaction 0 4 0 9 org 0 9 id 017 1 4 0 7 010144 0 0036 0 0018

Physical Chemistry
GeneralIn acidic medium H O2 converts PbS into X The behaviour of H O2 in the reaction and the product X so formed respectively are Reducing PbSO4 Oxidising PbSO4 Reducing SO2
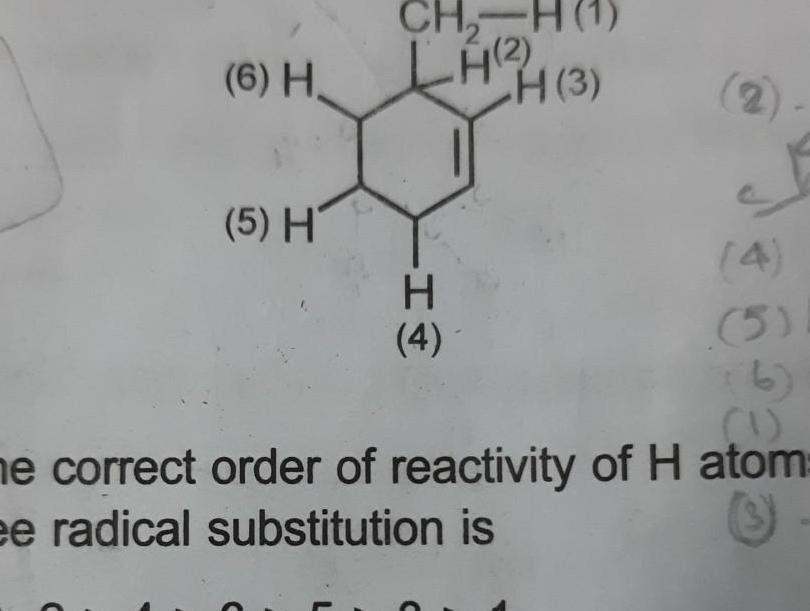
Physical Chemistry
General6 H 5 H CH LH 4 3 H 4 4 5 1 ne correct order of reactivity of H atom ee radical substitution is

Physical Chemistry
GeneralHow would you balance the following oxidation half reaction Fe s Fe aq You can either write out the balanced half reaction or simply state what and how many of somet you would add to either the product or reactant side

Physical Chemistry
ElectrochemistrySelect the ion of lowest limiting molar conductivity in water at 298 K 0 OH OC Br TYPE questions Each question has 4 choices 1 2 3 and 4 out of which only one is correct Na Read More

Physical Chemistry
SolutionsThe expression relating molarity M of a solution with its molality m is where d density Mg mol wt of solute 1000 M 1000d M MB 1 3 m m 1000d MMB M 2 m 4 m 1000 M 1000d M MB 1000d MMB 1000 M

Physical Chemistry
Energetics17 Estimate the enthalpy of combustion for methane at 298 K and 1bar using the bond enthalpies in the textbook Compare your result with that calculated from the enthalpies of formation of products and reactants CH g 20 g CO g 2H O g

Physical Chemistry
Solid stateA given metal crystallises out with a cubic structure having edge length of 361 pm If there are four metal atoms in one unit cell what is the radius of one atom a 80 pm c 40 pm C b 108 pm d 127 pm 2015 Cancelled