Chemical kinetics Questions and Answers

Physical Chemistry
Chemical kineticsDue to some accident a radioactive element spread over the laboratory its activity is found to be 10 times the permissible value The mean life of the radioactive element is 200 hours After how many days the room will be available for safe working 10 days 15 days 8 days 19 days

Physical Chemistry
Chemical kineticsMatch Column I with Column II Column I Q1 A B C r K A Q2 A B C 2D r K A B B Q3 A 2B C D r K A B Q4 2A BC Dr K A B Q1 03 A1 A2 A3 A4 A5 Column II A1 The rate of consumption of both the reactants is equal A2 Unit of rate constant is equal to 1 mol time A3 Rate of consumption of one of the reactant is twice of rate of formation of one of the product A4 t1 2 of both the reactants is equal if equal concentration is taken A5 t1 2 of one of the reactant is twice to that of the other reactant if equals concentration is taken

Physical Chemistry
Chemical kineticsThe following data were obtained at a constant volume for the decomposition of di tertbutyl peroxide in the homogenous gas phase at 500 K t min 0 10 20 P torr 200 250 290 The reaction is as following first order kinetics CH3 3 COOC CH 2CH COCH C H6 log 8 7 0 058 log 40 31 0 115 Identify the correct statement s C H6 At time equal to 10 min the pressure due to C H6 is 50 torr The rate constant is approximately equal to 1 33 10 min 1 At time equal to 20 min the pressure due to acetone is 90 torr The rato constant is ximatoly oqual to 0 8 x hr 1

Physical Chemistry
Chemical kinetics2 51 For a chemical reaction starting with some initial concentration of reactant A as a function of time t is given by the equation 2 1 5 x 10 t 1 Af The rate of disappearance of A is x 102 M sec when A 2 M Given A in M and t in sec Express your answer in terms of 102 M s Round off your answer if required

Physical Chemistry
Chemical kineticsWhen a gas is doubled through water at 298 K a very dilute solution of the gas is obtained Henry s law constant for the gas at 298K is 100 kbar If the gas exerets a partial pressure of 1 bar then number of millimoles of the gas dissolved in one litre of water is A 0 555 C 0 0555 B 5 55 D 55 5

Physical Chemistry
Chemical kineticsThe first step in the breakdown of methane is in the atmosphere is the reaction OH CH4 CH3 H2O Derive an expression for the lifetime of methane due to reaction with OH radicals by integrating the first order rate law Explicitly state assumptions you make in deriving the expression

Physical Chemistry
Chemical kineticsA sample of a radioactive substance undergoe 80 decomposition in 345 minutes Its half life minutes 1 3 In 2 In 5 In 5 In 4 x 345 345 2 4 In 5 x 345 In 2 In 4 In 5 345

Physical Chemistry
Chemical kinetics56 Reaction rate between two substances A and B is expressed as following rate k A B m the concentration of A is doubled and concentration of B is made half of initia concentration the ratio of the new rate to th earlier rate will be 1 m n 2 1 2 m n 3 2 n m 4 n

Physical Chemistry
Chemical kineticsFrom the concentrations of C H Cl butyl chloride at different times given below calculate the average rate of the reaction CH Cl H O during different intervals of time C H OH HCl t s 0 C H CI 0 100 mol L 50 100 150 200 0 0905 0 0820 0 0741 0 0671 t s 300 400 700 C H Cl 0 0549 0 0439 0 0210 mol L 1 800 0 017

Physical Chemistry
Chemical kineticsB For a reaction scheme A B2C if the rate formation of B is set to be zero then the concentration of A given by 2019 Main 8 Apri a k k A c k k 4 1 d k k A b A

Physical Chemistry
Chemical kineticsFor the decomposition of dinitrogen pentoxide at 200 C N 0 g N O g O g if the initial pressure is 114 mm and after 25 minutes of reaction the total pressure of gaseous mixture is 133 mm calculate the average rate of reaction in a atmosphere min and b mol L

Physical Chemistry
Chemical kineticsTue as 1st excitation energy of H atoms A hydrogen like atom in ground state absorbs n photons having the same energy and it emits exactly photons when electronic transition takes place Then the energy of the absorbed photon may be B 40 8 eV C 48 4 eV A 91 8 eV D 54 4 eV

Physical Chemistry
Chemical kineticsb According to the Collision theory for a reaction to occur between molecules there are three requirements that must met by molecules Mention these three requirements 3 The rate constant for a certain first order reaction is 0 40 min 1 i Calculate the initial rate in mol L min if the initial concentration of the compound involved is 0 50mol L 3

Physical Chemistry
Chemical kinetics5 In an acidic indicator HIn has ionization constant is 10 8 The acid form of indicator is yellow and alkaline form is red Which is correct statement bo Given log2 0 3 log3 0 48 a The pH range of indicator is 7 to 9 b Change in pH is 0 96 when 75 yellow colour change to 75 red colour c This indicator is suitable for the titration of strong acid vs strong base d pH of indicator is 8 3 when ratio of acid form to alkaline form is 2

Physical Chemistry
Chemical kinetics50 A gaseous reaction A g B g C g shows increases in pressure from 100 mm to 120 mm in 5 minutes The rate of disappearance of A is A 4 mm min 1 C 16 mm min 1 B 8 mm min 1 D 2 mm min 1

Physical Chemistry
Chemical kinetics58 If rate of reaction in by a factor of 2 when temperature is raised from 27 C to 37 C What is the value of activation energy in kJ mol By what factor does rate of reaction increased from 127 C to 137 C increase if temperature is Cherr Given In2 0 7 R 8 314 Joule mol K e0 4 1 5 a If temperature coefficient u is same in given temperature range b If temperature coefficient u is function of temperature

Physical Chemistry
Chemical kineticsD Consider the reaction A B The concentration of both the reactants and the products varies exponentially with time Which of the following figures correctly describes the change in concentration of reactants and products with time B B a c Concentration Concentration Time B Time A 4 b d Concentration Concentration Time Time 4 4 B

Physical Chemistry
Chemical kineticsElectrode potential for Mg electrode varies according to the equation 1 Mg Mg 2 log 2 Mg The graph of EMg2 Mg vs log Mg is EMg Mg a c EMg2 Mg EMg2 Mg E log Mg2 0 059 log Mg 1 b d EMg2 Mg EMg2 Mg log Mg2 Ing Mg 1

Physical Chemistry
Chemical kineticsThe decomposition of N O according to the equation 2N O5 g 4NO2 g O2 g is a first order reaction After 30 minutes from the start of the reaction in a closed vessel the total pressure developed is found to be 305 5 mm of Hg and on complete decomposition the total pressure is 587 5 mm Hg If the rate constant of reaction is xx 10 3 then the value of x is

Physical Chemistry
Chemical kineticsThe plot given below shows p T curves where p is the pres sure and T is the temperature for two solvents X and Y and isomolal solutions of NaCl in these solvents NaCl completely dissociates in both the solvents 760 Pressure mmHg 360 362 2 3 4 367 368 1 solvent X 2 solution of NaCl in solvent X 3 solvent Y 4 solution of NaCl in solvent Y Temperature K On addition of equal number of moles of a non volatile sol ute S in equal amount in kg of these solvents the elevation of boiling point of solvent X is three times that of solvent Y Solute S is known to undergo dimerisation in these solvents If the degree of dimerisation is 0 7 in solvent Y the degree of

Physical Chemistry
Chemical kinetics14 5 You can click on the Review link to access the section in your eText The data below show the concentration of N O5 versus time for the following reaction N O5 g NO3 g NO g Time s N O5 M 0 1 000 25 0 822 50 0 677 75 0 557 100 0 458 125 0 377 150 0 310 175 0 255 Part A Determine the order of the reaction n Submit VAX Request Answer Part B Complete previous part s DHE

Physical Chemistry
Chemical kineticsIf 60 of a first order reaction was completed in 60 minutes 50 of the same reaction would be completed in aproximately a 45 minutes c 40 minutes og 1 0 60 log 5 0 69 b 60 minutes d 50 minutes

Physical Chemistry
Chemical kineticsCalculate the approx ratio of for the 1st order 12 3 1 3 reaction T1 3 represents the time at which one thirds of the reactant is consumed log3 0 47 log 2 0 3 2 76 0 30 0 47 1 56

Physical Chemistry
Chemical kineticsA g B s C g The following data is given for the decomposition of A into B and C at 300 K and the reaction follows first order kinetics Time min 2050 Vol of C mL 1025160 It is given that vapour pressure of B s is negligible In16 2 77 In15 2 70 Identify the correct statement Rate constant of the reaction is 0 0035 sec 1 Rate of formation of B and C will be equal O Volume of C will be equal to volume of A at time 100 min Volume of C will never bogomo cual to volume of A

Physical Chemistry
Chemical kineticsonsider the following A g B s C g The following data is given for the decomposition of A into B and C at 300 K and the reaction follows first order kinetics Time min 2050 Vol of C mL 1025160 It is given that vapour pressure of B s is negligible In16 2 77 In15 2 70 Identify the correct statement Rate constant of the reaction is 0 0035 sec Rate of formation of B and C will be equal O Volume of C will be equal to volume of A at time 100 min

Physical Chemistry
Chemical kineticsCalculate the Ksp of BaCO3 when Na2CO3 is added slowly to a solution containing equimolar concentration of Ba and Mg2 and no precipitate is formed until 80 of Ba has been precipitated as BaCO3 Assume that the solubility of MgCO3 is 8 4 mg L A 4 0 x 10 9 B 2 0 10 9 C 2 4 x 10 10

Physical Chemistry
Chemical kinetics72 How many of the following is not correct 1 1 2 of zero order reaction is proportional to initial concentration of reactant ii 2 of first order reaction is independent of initial concentration of reactant iii t 2 of zero order reaction is equal to R 2K log2 k iv 1 2 of first order reaction is equal to v First order reactions will never go for completion

Physical Chemistry
Chemical kinetics67 Rate constant K varies with temperature by equation logk min 5 conclude that A Pre exponential factor A is 105 C Ea is 9 12 k cal 2000 kcal RTX 2 303 we can B Ea is 2 kcal D The pre exponential factor A is 5

Physical Chemistry
Chemical kinetics218 PO 11 2 183 sec decay to 82Pb tv2 sec by a emission while Pb2 4 is a B emitter In an experimental starting with 1 mole of pure Po2 8 how much time would be required for the number of nuclei of Pb to reach maximum

Physical Chemistry
Chemical kineticsA substance A gets converted to B following single step mechanism The variation of rate constant k vs temperature T is given by following equation 301 3 logio k 8 T From this information calculate fraction of activated particles at 1000 K Multiply your answer by 10 Given In2 0 693 In 10 2 3

Physical Chemistry
Chemical kineticsa 16 1 c 1 4 b 2 1 d 4 1 2015 Cancelled 1 What is the mass of the precipitate formed when 50 mL of 16 9 solution of AgNO3 is mixed with 50 mL of 5 8 NaCl solution Ag 107 8 N 14 0 16 Na 23 C1 35 5 a 3 5 g b 7 g c 14 g d 28 g 2015 If Avogadro number NA is changed from 6 022 1023 mol to

Physical Chemistry
Chemical kineticsThe pressure of a gas decomposing at the surface of a solid catalyst has been measured at different times and the results are given below t sec 0 100 200 4 103 Pr Pascal 3 5 10 3 10 Determine the order of reaction its rate constant 300 2 5 10

Physical Chemistry
Chemical kineticsIf an equimolar mixture of the two radioact substances having decay constant 5 303 hr and 3 hr respectively decay simultaneously Then ratio of nuclides 2nd with respect to 1st at the end of 2 hr is 10 P The value of P is Given In A 90 1 2 303 log Al

Physical Chemistry
Chemical kinetics56 What will be the order of reaction for a chemical change having logt 2vsloga where a initial concentration of reactant t 2 half life A Zero order log 2 B First order log a C Second order D None of these

Physical Chemistry
Chemical kineticsThe Enthalpy of formation of H SO4 at 298K will be Given S 02 SO SO2 1 2 02 SO3 SO3 H O H SO4 AH 300 KJ AH 100 KJ AH 130 KJ H 1 2 02 H O AH 280 KJ Question Type Single Correct Type 1 2 810 KJ 710 KJ

Physical Chemistry
Chemical kineticsi i Rate Concentration What is the order of the reaction What is the unit of rate constant K for the reaction Derive an expression to calculate the time required for completion of the zero orde

Physical Chemistry
Chemical kineticsRead the paragraph carefully and answer the The rate constant of reaction is related with T by Arrhenius equation Ea RT k A e B 3 Where k rate constant A Pre exponential factor or frequency factor Ea RT Fraction of molecules that is present in activated state ecules e E Activation energy Fraction of molecules that can cross the activation energy increases with increase in temperature or with decrease in activation energy conc Find the maximum rate constant A 3 46 x 102 day C 10 day SOLVE QUESTION 3 not 1 20 days Time R P Follows the above conversion graph and this first order reaction occurring at 27 C then if 102 molecules are in activated state 10 B 3 46 x 102 day D can t be determined In the question 1 of reaction is radioactive disintegration What should be its half life at 600 C A 20 days B 20 days

Physical Chemistry
Chemical kineticsThe following data are for the following decomposition of ammonium nitrite in aqueous solution Vol of N in cc 6 25 9 00 11 40 13 65 33 05 The order of reaction is a zero c two Time min 10 15 20 25 Infinity b one d three

Physical Chemistry
Chemical kineticsThe accompanying concentration of species A and B for the reaction AB as a function of time The point of inter section of the two curves represents B conc Times a c 2 3 1 2 d Data insufficient tom A b 13 4

Physical Chemistry
Chemical kinetics2 63 For the gaseous reaction A 1 dn A V dt Method I Where C Ca Products the rate may be expressed as dP nA V k Ch olar perts Method II A dt r concentration of A and P is the partial pressure of A t time t and n is the order of reaction The reaction is occurring at constant temperature T 300 K Assume ideal behaviour of gas Select the correct statement s A A k k for any value of n C k k RT when n 0 El 300 Experts k PA B k k when n 1 D k k RT when n 2

Physical Chemistry
Chemical kinetics3 For a reaction A B products the rate c reaction was doubled when concentration of A wa doubled When concentration of A and B both wa doubled the rate was again doubled order reaction w r t A and B are 1 1 1 3 1 0 2 2 0 4 0 1

Physical Chemistry
Chemical kineticsDecomposition of di 2 methyl propan 2 yl peroxide produces propanone and ethane CH3 3COOC CH 3 2 CH3COCH3 C H6 The generally accepted mechanism for the above decomposition is Step 1 CH3 3COOC CH3 3 2 CH3 3CO Step 2 Step 3 CH3 3CO CH3COCH3 CH3 CH3 CH3C H6 K K2 K3 a Write down the expressions for the rates of change of concentrations of the intermediate s and the product C H with respect to time 3 b Derive a differential rate law for the rate of formation of the product C H on the basis of

Physical Chemistry
Chemical kineticsSolveLancer Test Choose the correct statement for reaction 2A B zero order SolveLancer Test 1 Unit of k is moL L sec1 A o 2k 2 t A o k 3 Time required for 100 completion is 4 It may or may not be a complex reaction a All are correct b Only 1 2 and 3 are correct c Only 1 d None

Physical Chemistry
Chemical kineticsB g C g and half life for the first o Half life for the zero order reaction A g reaction X g Y g Z g are equal If completion time for the zero order reactio 13 86 min then calculate the rate constant in hr for the reaction X g Y g Z g

Physical Chemistry
Chemical kineticsnr Which of the following is not true regarding catalyst Catalyst does not change equilibrium constant value Coenzyme increases the catalytic activity of enzyme Catalyst can also catalyse non spontaneous reaction All of these

Physical Chemistry
Chemical kineticsall steps to be elementary K A 2A K A B AB B K4 B A AB A Which of the following is incorrect k3 O DA dt dB dt k A 2 k A K4 B A dB dt K3 A B k3 A B K4 B A dA 2 k A 1 2 k A1 k A B KIBA l

Physical Chemistry
Chemical kineticsDecomposition 3A g 2B g 2C g follows first order kinetics Initially only A is present in the container Pressure developed after 20 minute and infinite time are 3 5 and 4 atm respectively Which of the following is true A 150 20 min C 199 64 3 min B D t75 40 min 185 70 min

Physical Chemistry
Chemical kineticsIn the prior video we learned that the ratio of rate constants KA KB e AE RT where R 1 9872 cal mol K 1 Select the best answer for the product ratio A C at the end of the reaction if the the activation energy for B going to A is 4 2 Kcal mol higher than the activation energy for B going to C at room temperature 22 C 295 15 K and given the fact that the reactions of B going to A and B going C are both irreversible a A C 1 015 b A C 0 985 c A C 0 000776 d A C 1 288 A B KA KB e AE RT
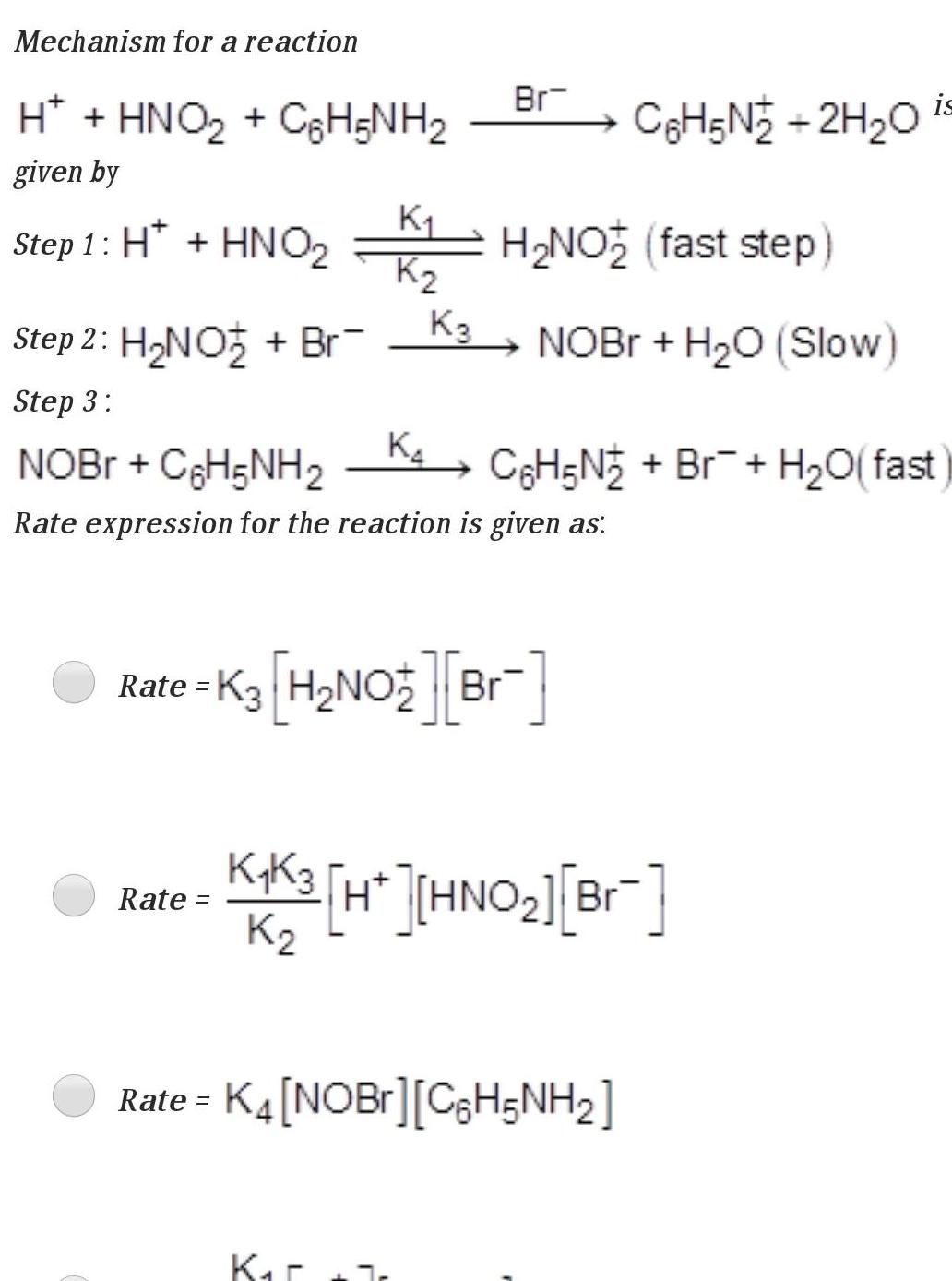
Physical Chemistry
Chemical kineticsMechanism for a reaction H HNOz CoH NH given by Step 1 H HNO2 Step 2 H NO Br Step 3 K K Rate K3 Br K NOBr C6H5NH Rate expression for the reaction is given as Kar H NO fast step NOBr H O Slow Rate K3 H NO Br is C6H5N 2H O C6H5N Br H O fast K4K3 H HNO Br K2 Rate K4 NOBr C H5NH 2

Physical Chemistry
Chemical kineticsConsider the following reaction A g B s C g The following data is given for the decomposition of A into B and C at 300 K and the reaction follows first order kinetics Time min 205000 Vol of C mL 1025160 It is given that vapour pressure of B s is negligible In16 2 77 In15 2 70 Identify the correct statement 0 Pate constant of reaction in 0 0035s Rate of formation of Band C will be equal Volume of C Volume of A at time 100 min