Chemical kinetics Questions and Answers

Physical Chemistry
Chemical kineticsA For a certain reaction A function of A is given as below A M 0 1 t 2 min 100 Which of the following is are true C 0 025 50 The order is 2 2 The order is 1 products the t 2 as a B D 1 2 would be 100 10 min for A 1M would be 100

Physical Chemistry
Chemical kinetics14 The dissociation of nitrogen pentaoxide is a first order reaction In first 12 min 75 of nitrogen pentaoxide is dissociated What amount of nitrogen pentaoxide will be left behind after one hour of the start of reaction 1 3 01 2 0 21 3 0 098 4 None of these

Physical Chemistry
Chemical kinetics8 The rate law of a reaction A B Product is rate k A B On doubling the concentration of A and halving the concentration of B the ratio of new rate to the earlier rate of reaction will be 1 n m 1 3 2m n 2 2n m 4 m n

Physical Chemistry
Chemical kinetics2 Which one of the following is wrongly matched 1 Saponification of CH3COOCH Second order reaction 2 Hydrolysis of CH3COOCH3 Pseudo unimolecular reaction 3 Decomposition of H O First order reaction 4 Combination of H and B to give HBr Zero order reaction

Physical Chemistry
Chemical kinetics1 In the formation of sulphur trioxide by the contact process 2SO 0 2SO3 the rate of reaction was measured as 3 0 104 mol Ls d 0 dt The rate of reaction expressed in terms of SO3 will be 1 3 0 104 mol Ls 2 6 0 x 104 mol L s 3 1 5 104 mol L s 4 4 5 x 104 mol I 1 S C

Physical Chemistry
Chemical kineticsQ 30 TE 4 1 00 26 For the reaction N 0 2NO 0 K N d NO dt Given d 0 dt 2 K N O and d0 K3 N O5 dt The relation in between K K and K is

Physical Chemistry
Chemical kinetics55 It takes 1h for a first order reaction to go to 50 completion The total time required for the same reaction to reach 87 5 completion will be a 1 75 h b 6 00 h c 3 50 h d 3 00 h

Physical Chemistry
Chemical kineticsQ 38 A first order reaction is 75 complete after 32 min when was 50 of the reaction completed O 16 min O 8 min 4 1 00 19 O 4 min
Physical Chemistry
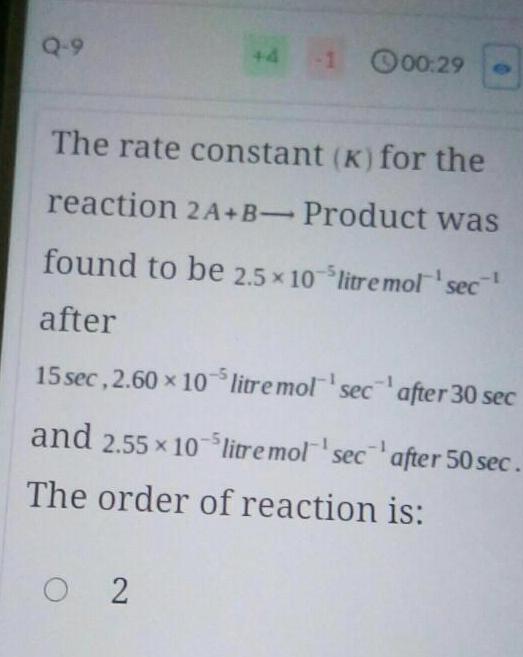
Chemical kineticsQ 9 4 00 29 The rate constant K for the reaction 2A B Product was found to be 2 5 10 litre mol sec after 15 sec 2 60 x 105 litre mol sec 02 after 30 sec after 50 sec and 2 55 10 litre mol sec The order of reaction is

Physical Chemistry
Chemical kineticsO 00 19 m n 0 The rate law for a reaction between the substances A and B is given by rate k A B On doubling the concentration of A and halving the concentration of B the ratio of the new rate to the earlier rate of the reaction will be as

Physical Chemistry
Chemical kineticsQ 16 O 4 Find the two third life 2 of a first order reaction in which k 5 48 x 10 4 per second 8 201 10 s 2 01 10 S O 201 10 0 s 00 47 0 201 10 s O

Physical Chemistry
Chemical kineticsQ 15 1 00 19 The rate constant is numerically the same for three reactions of first second and third order respectively Which one is true for rate of three reaction r r r

Physical Chemistry
Chemical kinetics20 When a graph between log k and 1 T is drawn a straight line is obtained The points at which the line cuts y axis and x axis respectively correspond to the temperatures 1 0 E 2 303R logA 3 0 logA 2 E R en A 4 log A

Physical Chemistry
Chemical kineticsConsider the following graph for the reaction A P AT 2 Lmol 8 tan 0 5 0 0 t min choose the correct statement s The given reaction is a second order reaction The given reaction is a third order reaction O Initial half life of reaction is 4 min Initial concentration of A is 2M

Physical Chemistry
Chemical kineticsQ 23 O 30s For a I order reaction A B the reaction rate at reactant concentration 0 01 M is found to be 2 0 10 Ms The half life period of the reaction is O 300s 4 1 C 00 05 220s O 000

Physical Chemistry
Chemical kineticsQ 3 O 0 04 DERATE 0 8 4 1 00 35 The rate constant for the reaction 2 N O 4NO O is 3 0 x 10 s If the rate is 2 4 x 105 mol L s then the concentration of N O in mol L is O

Physical Chemistry
Chemical kinetics4 A catalyst lowers the activation energy of a certain reaction at 27 C from 75 to 29 kJ mole If other parameters are same rate constant of the reaction 1 Increases by 108 times 2 Decreases by 104 times 3 Increases by 104 times 4 Decreases by 108 times

Physical Chemistry
Chemical kineticsQ 21 4 00 17 The time for half life period of a creation reaction A products is 1 h when the initial concentration of the reactant A is 2 0 molL how much time 4h does it take for its concentration to come from 0 50 to 0 25 mol L if it is a zero order reaction 1

Physical Chemistry
Chemical kineticsQ 7 4 1 O 72 times O 8 times 00 11 The reaction 2A B CD E is found to be first order in A second in B and zero order in C What is the effect on the rate of increasing concentration of A B and C two times 24 times O

Physical Chemistry
Chemical kineticsQ 13 04 Half life of a reaction is found to be inversely proportional to the cube of initial concentration The order of reaction is 03 4 85 1 00 10 388

Physical Chemistry
Chemical kineticsQ 5 4 1 Half life period of a first order reaction is 1386 seconds The specific rate constant of the reaction is O 5 0 10 00 16 05 0 10 s O 0 5 10
Physical Chemistry
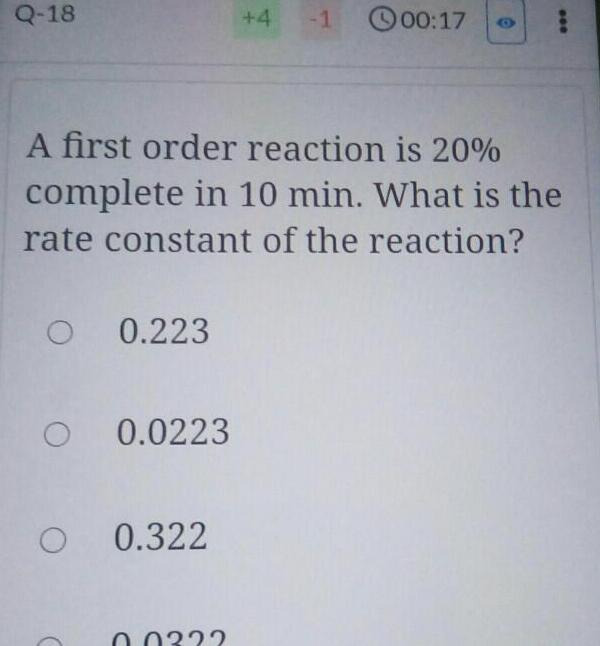
Chemical kineticsQ 18 O 0 223 O 0 0223 O 0 322 4 A first order reaction is 20 complete in 10 min What is the rate constant of the reaction 0 0332 1 00 17 000

Physical Chemistry
Chemical kineticsQ 8 O 8 min A reaction proceeds by first order 75 of this reaction was completed in 32 min the time required for 50 completion is O 16 min 4 1 00 23 O 20 min O 000

Physical Chemistry
Chemical kineticsQ 6 RATE 4 O 6 93 10 min The half life period of a first order reaction is 1 min 40 s Calculate its rate constant 6 93 10 5 1 06 93 10 s 00 14

Physical Chemistry
Chemical kineticsQ 34 4 1 The half life of two samples is 0 1 and 0 4 s Their reactive O 0 00 30 concentration is 200 and 50 respectively What is the order of reaction 02

Physical Chemistry
Chemical kineticsIn a first order reaction the concentration of the reactant is decreased from 1 0 M to 0 25 M in 20 minute The rate constant of the reaction would be O O 00 19 10 min 6 931 min

Physical Chemistry
Chemical kineticsClass XIII Spartan 56 For a second order reaction 2A products a plot of log 56 fife 34ff41 24 32414 an fet P t 2 vs log 1 a where a is initial concentration will give an intercept equal to which one of the following 3 log 1 a a 9 A 1 2 Joogl For the reaction 2 log 2k 4 log k 2A B3LD XY CE Bely StR Rexh 4 11 2 1 2 log 2k Log 41 12 log 2 love 4 log k 57 ffu fu

Physical Chemistry
Chemical kineticsQ2 Consider the titration of 30 0 mL of 0 015 0 M MnSO4 with 0 0100 M EDTA in a solution buffered to pH 8 00 Calculate pMn at the following volumes of added EDTA and sketch the titration curve a 0 0 mL b 15 0 mL c 30 0 mL d 45 0 mL e 60 0 mL f 75 0 ml

Physical Chemistry
Chemical kineticsneet prep Chemical kinetics Contact M 1 A hydrogenation reaction is carried out at 500 K If the same reaction is carried out in the presence of a catalyst at the same rate with same frequency factor the temperature required is 400 K What is the activation energy of the reaction if the catalyst lowers the activation energy barrier by 16 kJ mol 1 100 kJ mol 2 80 kJ mol 4 For first respectiv formation joule mol will be ob

Physical Chemistry
Chemical kineticsRate of appearance of D 58 The polential energy diagram for a reaction AB is 58 given below Energy A 1 x y 2 y z 3 x Z 4 W 7 Z X Biw R C The threshold energy of the reaction is

Physical Chemistry
Chemical kineticsAP min 1 0 0 5 0 25 me min 1 min 3 0 1 0 2 0 me min 1 of NOBR at Br at 27 C approx 40 Consider the reaction Reaction l P Q Reaction II Y Z First order reaction Rate constant K Activation energy X First order reaction Rate constant K Activation energy X Both reactions occur in different container In reaction l rate constant becomes 10 times to initial rate constant when temperature increases T to 3T while in reaction II rate constant becomes 10 times to initial rate constant when temperature increases T to 2T The correct statements is given x and x both are positive value and temperature independent T 300 K and value of Arrhenius parameter A of both reactions is same 1 X X 2 X X 3 If initially P Y 1 M at 300 K then rate of 1st reaction PQ is more than 2nd reaction Y Z 1 Roth 1 8 2 43 F

Physical Chemistry
Chemical kineticsThe correct statement regarding SO molecule is 1 two pr dr bonds 2 molecules has 2 lone pair 20 bonds and 2 bonds 3 two pr pr bonds 4 one px pr and one pr dr bond

Physical Chemistry
Chemical kineticshe rate constant for a first order reaction whose half life is 480 sec A skipped B 1 44 x 10 3 sec 1 D 1 44 x 102 sec 1 C 0 72 x 10 3 sec 1 2 88 x 10 3 sec 1

Physical Chemistry
Chemical kineticsFor a first order reaction A products the rate of reaction at A 0 2 M is 1 0 x 10 2 mol litre min The half life period for the reaction is 832 s 440 s 416 s 14 s

Physical Chemistry
Chemical kineticsthe half life period for catalyst decomposition of AB3 at 50 mm is found to be 4 hours and at 100 mm it is 2 0 hour The order of the reaction is A 3

Physical Chemistry
Chemical kineticson takes place in three steps with individual rate 55 constant and activation energy Rate constant Step 1 Step 2 Step 3 k k mak 1 140 kJ mol 3 130 kJ mol K K K3 overall activation energy of the reaction will be 2 150 kJ mol overall rate constant k Activation energy Ea 180kJ mol Ea 80kJ mol Ea 50kJ mol 2 3 K AC A

Physical Chemistry
Chemical kinetics67 Half life time of the following first order reaction is 20 min 6 A g B g C g If initial pressure of A g is 1 atm then the total pressure of system after 80 min is 1 1 5 atm 2 1 75 atm 3 1 93 atm 4 2 5 atm inte 15 16

Physical Chemistry
Chemical kinetics3 A first order reaction at 300 K temperature takes about 75 completion of 20 Takes minutes When the same reaction was done at 300x then what percent of the reaction will be completed in 40 minutes R 8 K mole In 2 0 7 1 12 5 2 93 75 06 25 4 87 5

Physical Chemistry
Chemical kineticsThe increase in the rate of a reaction with increase in temperature is mainly due to B the increase in collision frequency b the decrease in activation energy c the increase in number of activated molecules d the increase in pre exponential factor h

Physical Chemistry
Chemical kinetics6 The decomposition of N O in carbon tetrachloride was followed by measuring the volume of O gas evolved 2N 0 CC1 2N O4 CC14 O g The maximum volume of 0 gas obtained was 100 cm In 500 minutes 90 cm of O were evolved The first order rate constant in min for the disappearance of N 0 is 2 303 2 303 b log c 500 500 2 303 100 500 90 log 90 100 d 100 10 500 TEST Platinum fe c 63 Th 8

Physical Chemistry
Chemical kineticsc linear increase with increase of temperature d linear decrease with increase of temperature The plot of log k vs I T is linear with a slope of a Ea R b Ea R C the c Ea 2 303R a Ea 2

Physical Chemistry
Chemical kinetics15 The activation energy in a chemical reaction is defined as the difference in average energies of reactants and products b the difference in average energies of reactants and activated complex c the difference in average energies of products and activated complex d the sum of energies of rea tants and products

Physical Chemistry
Chemical kineticsThe rate constant of an exothermic reaction follows exponential increase with increase of temperature b exponential decrease with increase of temperature c linear increase with increase of temperature d linear decrease with increase of temperature The plot of log k vs T is linear with a slope of

Physical Chemistry
Chemical kineticsFor thermal decomposition of NH4NO3 s The correct information s is are NH4NO3 s AH N O g 2H O g at TK Patm Heat of reaction is positive Entropy change of the reaction must be A H T Heat of reaction at constant volume is equal to A H 3 RT DAG of this reaction may be negative at high temperature

Physical Chemistry
Chemical kineticsa zero 6 integral c fractional a negat 5 If the activation energy of a reaction decreases then the rate of reaction a decreases b remains constant increases d becomes zero 7 The rate constant of a first order reaction depends on the

Physical Chemistry
Chemical kineticsThe initial concentration of the reactant in a zero order reaction is 1 386 mol L 1 The half life of the reaction is 20s Calculate the rate constant 2 the concentration of the reactant after 30s from the initiation of the reaction

Physical Chemistry
Chemical kinetics5 For a reaction 2A B A B then which of the following is correct 1 the unit of k is second 2 tis constant 3 the rate of formation of C is twice to that of rate of disapperiance of A 4 the value of k is not depend upon concentration of A and B A first order roactic 7500 C the rate law is rate k 5 37f4f 2A B C A B 1 kansass Fred Kinetics Periodic Tal Cafe aff q 2 5 2 3 C 49 A faqtisku sasa it he 4 R Gur 9 Fo re

Physical Chemistry
Chemical kineticsslow N fast NO F F NO F NO F NO F 341 34fell disch ch fay yar fac 2 1 r k NO F 2 K NO F 3 F k NO 4 r k F at f 22 d ve 202 at d 1 dl 2 I lady

Physical Chemistry
Chemical kinetics8 Exp No 1 2 The following data are for the reaction A B Product 3 4 Concentration 4 0 x 104 1 6 x 10 1 0 x 10 1 0 x 10 The correct statement s regarding given reaction is are a Overall order of reaction is 2 b The rate of reaction is independent of concentration of B of A M 0 10 0 20 0 50 0 50 3 a c 4 a b c Concentration of B M 0 10 0 20 0 10 0 50 Rate MS c The rate constant is 4 x 10 2 L mol S 1 a b 2 b c 8 4 2 Exp No 1 2 3 4 fufhalt folg fysis fed Concentration Concentration Rate MS of B M of A M 0 10 0 10 24 0 20 0 20 0 50 0 10 0 50 0 50 4 0 x 10 a life 2L b B Revision Test Series PTS for NEETUL2015 1 6 x 109 1 0 x 102 1 0 102 c Fai 4 x 102 L mot S U 1 a b 2 b c 3 a c 4 a b c The 12 A 4X10 2 k 0 Tor 2 Te b

Physical Chemistry
Chemical kinetics35 In the reversible reaction 2 NO disappearance of NO is equal to 1 NO 1 2k k 2 2k NO 1 2 N 04 3 2 k NO 1 K N 04 4 2 k k NO k 4 1 k N O4 the rate of 344 3442 NO 1 2k1 NO k 2 2k NO 1 2k 0 3 2k NO K N 04 4 2 k k NO sese 2 00 N O4 NO fac Id Care 2 at of N 7 NOL