Energetics Questions and Answers

Physical Chemistry
Energetics4 PH3 2 H2 902 2 P 05 The reaction has AG 25 kJ or AG 25 kJ The reaction is exergonic or endergonic The reaction is spontaneous or non spontaneous c absorbs heat a exothermic g endergonic I AG 25 kJ b endothermic h AG 25 kJ 8 H O liq 25 kJ d gives off heat j spontaneous k non spontaneous e none of these choices W f exem

Physical Chemistry
EnergeticsCalculate AH for the following reactions using the heats of formation remember all diatomics and single elements are 0 kJ mol a 8Al s 3Fe3O4 s 4Al2O3 s 9Fe s

Physical Chemistry
EnergeticsHow much energy is required to increase the temperature of 56 00 g of water from 1 000 C to 21 00 C The specific heat capacity c of water is 4 18 J g C Helpful equation q m c AT 4 682 g 4 682 C 418 J 4 682 J

Physical Chemistry
EnergeticsChoose the correct answer Congress first levied an income tax to pay for O early growth of the federal government O the Civil War O programs after the Great Depression

Physical Chemistry
EnergeticsMolecular systems tend to move spontaneously to a state of maximum randomness or disorder Molecular randomness or disorder is called entropy and is denoted by the symbol S As a state function entropy change AS depends only on initial and final states AS has a positive value when disorder increases and a negative value when disorder decreases The following conditions usually result in an increase in entropy a change of phase solid liquid gas an increase in the number of gas molecules or a solid dissolving to form a solution Although the sign of the entropy change can be predicted as described above the actual value of AS must be calculated from the absolute entropy values S of the reactants and products AS S products S reactants Submit Part B Calculate the standard entropy change for the reaction using the data from the following table 2Mg s O g 2MgO s Substance AH kJ mol AG kJ mol S J K mol Mg s 32 70 O2 g MgO s A 0 00 0 00 602 0 0 00 0 00 569 6 205 0 Express your answer to four significant figures and include the appropriate units View Available Hint s 27 00

Physical Chemistry
Energeticspose the vapor pressure of a substance is asured at two different temperatures Part A AHvap RT By using the Clausius Clapeyron equation In P pressures P and P2 and the absolute temperatures at which they were measured T and T Express your answer in terms of T T2 AHvap and the gas constant R In P P2 Submit Part B VAE Request Answer PSS2 VAE Gasoline is a mixture of hydrocarbons a major component of which is octane CH3 CH CH CH CH CH CH CH3 Octane has a vapor pressure of 13 95 torr at 25 C and a vapor pressure of 144 78 torr at 75 C Use these data and the equation in part a to calculate the heat of vaporization of octane Express your answer using two significant figures 44 C derive the relationship between the vapor

Physical Chemistry
Energetics3 The density of a metal is 2 12 grams per mL What is the volume of 45g of the meta

Physical Chemistry
Energetics3 points Given the following combustion reaction C6H12O6 s 6 O2 g 6 CO g 6 H O g and the enthalpy of formations in kJ mol for each species are C6H12O6 s 1273 3 CO2 g 393 5 and H O g 286 0 Determine the heat exchange in kJ when 29 6 g of C6H12O6 s molar mass 180 16 g mol is burned in excess oxygen Answer to 1 decimal place

Physical Chemistry
EnergeticsMetal Calcium Iron Silver Gold Specific Heat J g C Gold 0 647 0 449 0 235 0 129 After absorbing 0 500 kJ of energy a 55 5 g sample of an unknown substanc temperature increase of about 14 degrees Celsius Identify the substance The substance is not listed

Physical Chemistry
EnergeticsCarrie is trying to figure out the number of calories in a cube of cheese To do this she pours 149 5 mL of water into an aluminum can suspended from a ring stand She takes the temperature of the water and finds it to be 17 8 degrees Celsius Then she places the 5 23 gram cube of cheese under the can and lights it on fire While the cheese is burning and for a few minutes after it is done Carrie records the temperature of the water finding that it levels out at 21 1 degrees Celsius How many calories of heat were gained by the water Please answer to the nearest 0 1 calorie Your Answer

Physical Chemistry
Energetics3 points When 6 84 g of hydrocarbon molar mas 314 1 g mol is burned in a bomb calorimeter the calorimeter increases in temperature by 2 95 C If the heat capacity of the bomb calorimeter is 1 071 kJ C what is the heat of combustion for the hydrocarbon in kJ mol Answer to 1 decimal place Type your answer

Physical Chemistry
EnergeticsWhich of the following statements are TRUE During a phase change temperature does not change O During a phase change temperature decreases During a phase change temperature does change During a phase change temperature increases

Physical Chemistry
Energetics4 PH3 2 H 90 2 P 05 The reaction is endothermic or exothermic The reaction gives off heat or absorbs heat a exothermic b endothermic g endergonic h AG 25 kJ 8 H O liq c absorbs heat I AG 25 kJ 25 kJ d gives off heat e none of these choices j spontaneous k non spontaneous f exerg

Physical Chemistry
EnergeticsWhat is the boiling point of the substance modeled by this heating curve Temp C 150 135 120 105 90 75 60 45 30 A 15 B Created by E Lee for Virtual Virginia 2021 C D Heat kJ E F

Physical Chemistry
EnergeticsAccording to Thomas Paine in his essay Common Sense What if delayed longer will be harder to accomplish Overthrowing the government O Independence of America Establishing a republic Reconciliation
Physical Chemistry
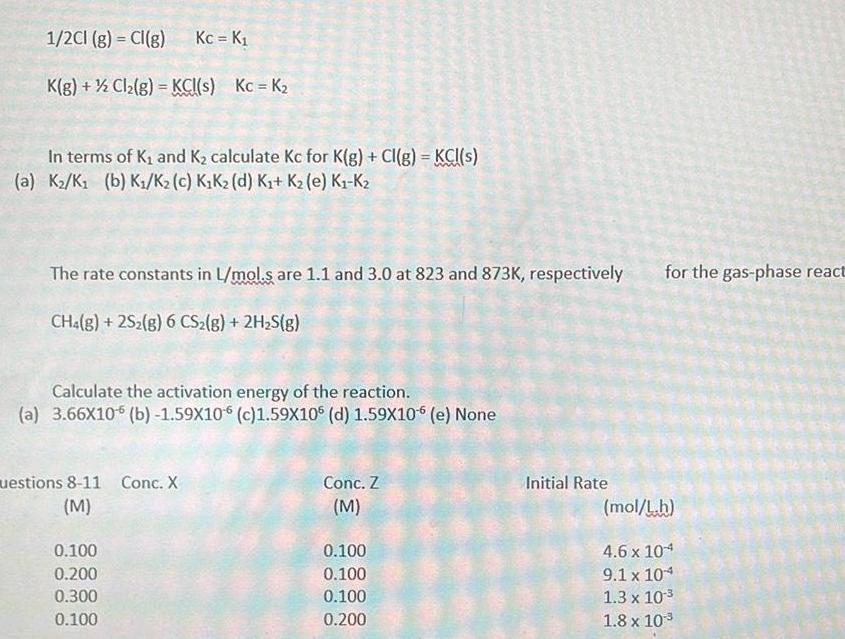
Energetics1 2Cl g Cl g K g Cl g KCl s Kc K In terms of K and K calculate Kc for K g Cl g KCl s a K K b K K c K K d K K e K K Kc K The rate constants in L mol s are 1 1 and 3 0 at 823 and 873K respectively CH4 g 2S2 g 6 CS g 2H S g Calculate the activation energy of the reaction a 3 66X10 b 1 59X106 c 1 59X106 d 1 59X10 6 e None uestions 8 11 Conc X M 0 100 0 200 0 300 0 100 Conc Z M 0 100 0 100 0 100 0 200 Initial Rate for the gas phase react mol L h 4 6 x 104 9 1 x 104 1 3 x 10 1 8 x 10

Physical Chemistry
EnergeticsD A helicopter takes off from the roof of a hospital and rises After reaching its peak it flies several miles at constant height to a park where it lands to pick up a patient Height of the Helicopter 0 Time Height of the Helicopter 0 Time Height of the Helicopter 0 O Time x Height of the Helicopter Time

Physical Chemistry
EnergeticsWhen the reaction CH3CI g H O g CH3OH g HCl g was studied the tabulated data were obtained Based on these data what are the reaction orders Exp CH3CI 1 0 100 2 0 200 3 0 200 4 0 200 H O Initial Rate M s 0 100 0 200 0 400 A CH3CI first order and B CH3Cl first order and order 0 100 0 182 1 45 5 81 0 364 H O first order H O second C CH3Cl second order and H O first order D CH3CI second order H O second order

Physical Chemistry
EnergeticsFor the decomposition of mercury II oxide HgO to mercury and oxygen 2HgO s 2Hg 1 O g AG 117 1 kJ What is the standard Gibbs free energy of formation AGf of mercury II oxide A 117 1 kJ mol B 58 6 kJ mol C 58 6 kJ mol D 117 1 kJ mol

Physical Chemistry
Energeticseto 7 How much heat in joules and in calories is required to How much would the temperature of 275 g of water increase if 36 5 kJ of heat were added If 145 kl of heat were added to 485 g of liquid water how much would its temperature in ixo 3110 I to increase its temperat

Physical Chemistry
EnergeticsGenerally which process in the multistep conversion of a solid to a gas requires the most energ O Heating the liquid from the freezing point to the boiling point O Vaporization of the liquid at the boiling point O Melting the solid to the liquid O Heating the solid to the melting point

Physical Chemistry
EnergeticsWrite the balanced chemical equation for the complete combustion of propionaldehyde C3H60

Physical Chemistry
EnergeticsWhich of the following is a heat of formation reaction C diamond O2 g CO2 g O C graphite O2 1 CO2 g 2C graphite 202 g 2CO2 g OC graphite O2 g CO2 g

Physical Chemistry
Energetics26 Given the reaction 2NH4NO3 s Ba OH 2 s 2H O 2NH3 Ba NO3 2 s AH Ran 196 kJ and relevant AH values calculate the heat of formation of NH4NO3 Show work 7 Don t look at the thermodynamic values table AH values Ba OH 2 s 946kJ H O 286 kJ NH3 g 46 kJ Ba NO3 2 s 990 kJ mol

Physical Chemistry
EnergeticsThe enthalpy of vaporization of Substance X is 29 0 Round your answer to 2 significant digits atm kJ and its normal boiling point is 29 C Calculate the vapor pressure of X at 78 C mol

Physical Chemistry
Energetics7 What is the second law of thermodynamics Normal bCcDdEe AaBbCcL Heading 1 9 Why is Gibb s free energy usually more useful to chemists than Helmhol No Spacing 8 What is the third law of thermodynamics Explain how this makes entropy different than energy or enthalpy

Physical Chemistry
EnergeticsJ The decarboxylation of pyruvic acid occurs via the following reaction CH COCOOH 1 CH CHO g CO g Given the following thermodynamic data A H 25 C CH COCOOH 584 kJ mol A H 25 C cu cao 166 kJ mol l A H 25 C co 394 kJ mol AaBbCcDdEe Normal lac b Calculate the equilibrium cortant Kp for this reaction at 80 0 K AaBbCcDdEe No Spacing A G 25 C CH COCOOH 463 kJ mol A G 25 C ch cho 133 kJ mol A G 25 C co 394 kJ mol At the lower temperature does the reaction favor the reactants or the products a Calculate AG Is this reaction spontaneous under standard state conditions Justify your answer AaBt Headira

Physical Chemistry
EnergeticsIf the body has a mass of 10 0Kg calculate 14 A body falling from a height of 100m drops into a mug of water the kinetic energy of the ball just as it strikes the surface of the water Assume g 9 81 m sec a 981 J b 9810 0J c 0 981J d 98 1J e 0 981KJ 15 The heat capacity of an object is 10 0 cal mol k If the object is made of iron and its mass is 2 0 g calculate the heat liberated by the object if its temperature changes from 15 C to 25 C At Wt Fe 55 85g mol a 2001 b 3 58 J c 101 3 J d 3 58 cal e None

Physical Chemistry
Energetics2 Mg s O2 g 2 MgO s A Positive because there is a solid as a product B Positive because there are more moles of reactant than product C Positive because it is a synthesis reaction D Negative because there are more moles of gas on the reactant side than the product side Juu of 61 F Sunny

Physical Chemistry
EnergeticsIn this experiment the following reaction enthalpies were determine 1 NaOH s NaOH aq AH 41 9 kJ 2 NaOH s HCl aq NaCl aq H O 1 AH 103 4 kJ Use Hess s law to determine the reaction enthalpy of 3 NaOH aq HCl aq NaCl aq H O 1 AH kJ


Physical Chemistry
Energeticspri 0 of 2 points earned 2 attempts remaining MM solution of HN3 Ka 1 9 10 x 7 For cyclohexane the AH of vaporization is 33 10 kJ mol and the AS of vaporization is 111 80 J mol K At 1 00 atm and 201 K what is the AG of vaporization for

Physical Chemistry
EnergeticsUse the information below to answer questions 11 12 that follow Show ALL work to receive full credit Wood is mainly cellulose a polymer produced by plants One use of wood is as a fuel in campfires fireplaces and wood furnaces The molecules of cellulose are long chains of repeating units Each unit of the chain can be represented as C6H100s The unbalanced equation below represents a reaction that occurs when C6H100s is burned in air C6H1005 0 CO H O heat 11 1 pt Balance the equation above Write the coefficients in the lines provided C6H1005 0 CO H O heat CA FA 88712 12 4 points How many moles of oxygen gas would be required to burn an entire log containing a mass of 76 9 grams of cellulose Show ALL work here

Physical Chemistry
EnergeticsHow much heat will be given off if molar quantities of cyclopropane react according to the following equation 2 C3H6 9026 CO 6H O

Physical Chemistry
EnergeticsConsider the following reaction at 25 C 3 Ni s N g 3 H O g 3 NiO s 2 NH3 g Given the information in the table calculate AS for the reaction Compound AHf NiO s Ni s NH3 g N g H O g kJ mol 239 7 0 46 0 241 8 Sf J mol K 38 29 9 192 5 191 5 188 7

Physical Chemistry
EnergeticsWhich pair of phenomena both support the particle model of light O A Diffraction and interference O B Blackbody radiation and diffraction OC The photoelectric effect and constructive interference D Blackbody radiation and the photoelectric effect

Physical Chemistry
EnergeticsCH4 g 2O2 g CO2 g 2H O 1 AH 890 kJ mol Calculate much heat is released when 3 5 moles of methane gas undergo a combustion reaction 4 Hess s Law 5 pts Use the word bank below to complete the statement Not all of the words will be used Heat sum AH Temperature Enthalpy Moles total AH

Physical Chemistry
EnergeticsCalculate the AHxn for the theoretical reaction of 2A B C Given C 2A D B D O 250 kJ 150 kJ O 150 kJ 250 kJ AHrxn 50 kJ AHrxn 200 kJ

Physical Chemistry
EnergeticsHow many kilojoules are released when 100 g of NO reacts This reaction is 2NO 202 2NO Select one O a 190 kJ endothermic O b 380 kJ exothermic O c 380 kJ endothermic O d 190 kJ exothermic AH 114 kJ

Physical Chemistry
EnergeticsThe heat capacity of liquid water is about 4 J g C and the heat capacity of ice is about 2 J g C Which of the following would have the smallest change in temperature if 100 J of heat was added O 20 grams of ice O 20 grams of water O 10 grams of water 10 grams of ice

Physical Chemistry
EnergeticsWhat is the work done when a volume increases from 3 liters to 6 liters at 2 atm of pressure Conversion factor 1 liter atm 100J 600 J of work done on the system 600 J of work done by the system O 6J of work done on the system 6 of work done by the system

Physical Chemistry
EnergeticsWater has a heat of vaporization of about 2000 J gram and a heat capacity for steam of about 2 J g C How much heat is needed to warm a 10 g of liquid water at 100 C to 150 C 210 kJ 201 kJ 21 kJ 20 kJ

Physical Chemistry
EnergeticsSelect or type in the BEST answer for each of the following questions You may use a calculator and scratch paper Be sure to turn your scratch paper in to potentially receive partial credit points 3 points 131 grams of an ethanol solution that is initially at 18 9 C absorbs 1 948 J of heat What will the final temperature Tf be for this ethanol solution The specific heat of ethanol is 2 42 J g C Just type your final answer in rounded to one 1 decimal NO units typed in

Physical Chemistry
EnergeticsA non spontaneous reaction can never be done in a lab but they may exist in nature O True False

Physical Chemistry
EnergeticsWith change in enthalpy as negative and change in entropy as positive what is gibb s free energy positive negative

Physical Chemistry
Energetics26 2 points A bomb dropped from an airplane just before it hits the ground Does this have more kinetic or potential energy potential O kinetic Previous

Physical Chemistry
EnergeticsSubstance C is identified to have a specific heat capacity of 0 452 3 gC Which substances have a higher specific heat capacity than Substance C Assume the masses of each substance are the same 9 meAT Change in Temperature vs Heat Added Temp C Heat Energy Added kJ Cmoted by E Lee for Virtual Virginia 2021 D

Physical Chemistry
Energetics5 2 points The specific heat of gold is higher than the specific heat of water True False O O Previous

Physical Chemistry
EnergeticsPlease answer the following questions based upon Chem Reading Enthalpy and Heats of Formation Reaction 4 Explain the difference between a positive enthalpy change and a negative one 5 What is the symbol for a change in enthalny

Physical Chemistry
Energetics1 point A food item contains 23 grams of carbohydrates 14 grams of fat and 5 grams of protein what is the total energy content for this food item in kcal Type your exact answer in with NO units D