Equilibrium Questions and Answers

Physical Chemistry
EquilibriumWhat are the Br nsted Lowry bases in this reaction NH3 aq H2O I NH aq OH aq A NH3 and OH B H O and NH4 C NH3 and H O D NH4 and OH C

Physical Chemistry
EquilibriumIndicate whether the following substances are strong acids weak acids neutral weak bases or strong bases base on their pH a b c d Baking soda pH 8 Liquid plumber pH 12 Pepsi pH 2 6 Pickle juice pH 5 e f g h Lye pH 13 Ajax liquid pH 7 8 Nail polish Remover pH 6 5 Purified water pH 7

Physical Chemistry
EquilibriumHd 10 9 4 13 12 11 8 6 5 7 1 0 3 2 0 A student is to determine the concentration of an NaOH aq solution by performing a titration with 25 0 mL of 0 100 M HCI aq with a sample of NaOH aq The volume of the NaOH aq added and the corresponding pH value of the reaction mixture is measured The graph below represents the relationship between pH and the volume of the NaOH aq added for the titration pH versus Volume of NaOH aq Added to HCl aq 5 10 15 20 25 30 35 40 45 50 Write a balanced chemical equation for the neutralization reaction that occurs

Physical Chemistry
EquilibriumNO g Cl g NOCI g Cl g s found to have an equilibrium constant of 1 1 108 at a particular temperature What does this mean A At equilibrium there is a much higher concentration of reactants than products B At equilibrium there is a much higher concentration of products than reactants C At equilibrium there is an equa concentration of reactants and products D At quilib

Physical Chemistry
EquilibriumQuestion 2 4 pts x 5 In the hypothetic reaction 2A s 3B aq C g 2D g energy What is this reaction s equilibrium constant expression If more A is added to the reaction system how the equilibrium will be affected Explain your answer i i ii If more D is added to the reaction system how the equilibrium will be affected Explain your answer iii If the system temperature is increased how the equilibrium will be affected Explain your answer iv If a catalyst is added to the reaction system how the equilibrium will be affected Explain your answer

Physical Chemistry
EquilibriumQuestion 4 A solution contains 0 04 M of sodium hydroxide What s this solution s molarity of hydrogen ion Molarity of hydroxide ion The solution s pH And pOH Is this solution acidic or basic

Physical Chemistry
EquilibriumIf we slowly add a solution of mercury II ions to a solution of aqueous halide ions with roughly equal concentrations a precipitate will form Explain what the precipitate will consist of initially Select the correct answer below The initial precipitate will consist of a single compound HgX where X is the halogen for which HgX has the smallest Ksp O The initial precipitate will consist of a single compound HgX where X is the halogen for which HgX has the largest Ksp The initial precipitate will consist of a mixture of halide compounds of the form HgX with mole fractions proportional to their respective Ksp values The initial precipitate will consist of a mixture of halide compounds of the form HgX with mole fractions inversely proportional to their respective Ksp values

Physical Chemistry
EquilibriumD 1 point When two elements react to form more than one compound a fixed mass of one element will react with masses of the other element in a ratio of small whole num True False

Physical Chemistry
EquilibriumWhen the following chemical equation is balanced what are the coefficients a b c and d a K S b CuCl c KCI d CuS Oa 2 b 2 c 2 d 2 Oa 0 b 0 c 2 d 0 Oa 1 b 2 c 1 d 2 O a 1 b 1 c 2 d 1

Physical Chemistry
EquilibriumUse Le Chatelier s Principle to decide what happens to the PH3 All are gases 4 PH3 2 H 90 2 P 05 8 H O 25 kJ PH3 when the temperature is increased PH3 when the temperature is decreased PH3 when the pressure is increased PH3 when the pressure is decreased c is changed EXVEENTE AURE hstant HUR MA a increases b decreases

Physical Chemistry
EquilibriumGive ways that could be used to increase production of P2O5 in the reaction if all are gases 4 PH3 2 H 902 2 P 05 8 H 0 25 kJ we want the reaction to go PH3 H 0 H O the temperature the pressure add a catalyst since the reaction is no longer at equilibrium a increaso H h

Physical Chemistry
Equilibrium2NO2 g N O4 g AH rxn 0 If the temperature is increased by 20 K while the volume is kept constant in which direction will the equilibrium shift V A reactants B product C neither the reactants nor the product hip 54 F Cloudy 1 09 PM 5 3 2022

Physical Chemistry
EquilibriumN O4 g 2NO2 g The AH for this reaction is 0 If the temperature is increased by 20 0 K while the volume is kept constant in which direction will the equilibrium shift A reactant B product C no change a 54 F Cloudy 1 10 PM 500022

Physical Chemistry
EquilibriumIf Kp 947 for the reaction below at 310 0 K then what is the value of Kc Note Kc is sometimes called K R 0 0821 L atm mol K 2 A g B s 2 C s D g

Physical Chemistry
EquilibriumThe following equilibria were attained at 823 K CoO s H g Co s H O g K 67 CoO s CO g Co s CO2 g Kc 500 Part A Based on these equilibria calculate the equilibrium constant for H2 g CO2 g CO g H O g at 823 K Express your answer using two significant figures Kc AEO

Physical Chemistry
EquilibriumA generic salt AB3 has a molar mass of 323 g mol and a solubility of 5 20 g L at 25 C What is the Ksp of this salt at 25 C AB3 s A aq 3B aq

Physical Chemistry
EquilibriumWrite the expression for the Ka when HC H3O2 is dissolved in water HC H3O H O C H30 H30 K a acetic g sulfurous n HPO4 u H O b hydrochloric c phosphoric h hydrosulfuric i OH j H30 k SO4 o H PO41 p CO3 S HS 1 v H CO3 W PO43 q HCO3 X HCzH3O2 d perchloric e hydrofluoric 1 Mg 2 t S 2 r H S y C H30 f sulfu m ch

Physical Chemistry
Equilibrium1 mole 6 02x1023 molecules Key Equations 1 mole 6 02x102 atoms ne the balanced chemical equation for the decomposition of nitrogen trichloride

Physical Chemistry
EquilibriumProblem 4 Write the product of an acid base reaction below 6 pts HF NaCH3CO which way does the equilibrium lie Problem 5 The pH Loop 8 pts Please complete the following table Report all concentrations to at least 3 significant figures Use scientific notation for the concentrations Report the pOH to the hundredth s place Show your work pH H or H3O OH pOH 9 50 Iced tea

Physical Chemistry
Equilibrium1 Warm up An aqueous solution of sodium hydroxide is combined with a solution of copper II acetate forming a precipitate of copper II hydroxide and an aqueous solution A Write a balanced chemical equation for the chemical reaction B Write a complete ionic equation for the chemical reaction C Write a net ionic equation for the chemical reaction 2 Read Chapter 14 Section 1 and 2 take notes and define the vocabulary in your notebook 3 Homework

Physical Chemistry
Equilibriumthe pH of a soluti 9 Calculate the pH of each of the following strong acid solutions a 8 5 x 10 3 M HBr b 1 52 g of HNO3 in 575 mL of solution c 5 00 mL of 0 250 M HCIO4 diluted to 50 0 mL

Physical Chemistry
Equilibrium6 If you have start with 10mL of a 1 M HCl solution and dilute it what is the H and pH of that solution a Using M1V1 M2V2 C1V1 C2V2 If you dilute it to 100 mL what is the new H and pH b What if you dilute the original sample to 1000 mL C What conclusion can you make about the relationship between changes in H and changes in pH 7 A sample of blood as a H 4 60 x 108 mol dm Calculate the concentration of OH and state whether the blood is acidic basic or neutral

Physical Chemistry
Equilibrium33 0 mL sample of a 0 439 M aqueous acetic acid solution is titrated with a 0 250 M aqueous sodium hydroxide solution What is the pH after 33 4 ml of base ave been added

Physical Chemistry
Equilibrium11 Calculate OH and pH for 1 5 x 10 M strontium hydroxide b 2 250 g of lithium hydroxide in 250 0 mL of solution C 1 00 mL of 0 175 M sodium hydroxide diluted to 2 00 L

Physical Chemistry
EquilibriumFill in the left side of this equilibrium constant equation for the reaction of hydrocyanic acid HCN with water 0 K 00 X Olo


Physical Chemistry
EquilibriumIf a solution has a pH of 2 what is the hydroxide ion concentration 2 1x10 12 M 1x10 2 M 12

Physical Chemistry
EquilibriumIf the rate of the forward reaction is equal to the rate of the reverse reaction the reaction is said to be O Finished at Equilibrium O at Rest Not started yet

Physical Chemistry
EquilibriumWhy do we balance chemical equations Law of Conservation of Charge Law of Conservation of Energy Law of Conservation of Momentum Law of Conservation of Mass VENTARICAIS AKERS

Physical Chemistry
EquilibriumA galvanic cell consists of a Cu s Cu2 aq half cell and a Zn s Zn aq half cell connected by a salt bridge Oxidation occurs in the zinc half cell The cell can be represented in D standard notation as Cu s Cu2 aq Zn s Zn aq Zn s Zn aq Cu s Cu aq Zn aq Zn s Cu s Cu aq Cu2 aq Cu s Zn s Zn aq Zn s Zn2 aq Cu aq Cu s

Physical Chemistry
Equilibrium2 points True or False The reaction S O82 Ni OH 2 2OH2SO42 NiO 2H2O will occur spontaneously under standard state conditions Following are the standard reduction potentials that may be useful NiO2 2H O 2e Ni OH 2 2OH E 0 49 V S 08 2e 2SO4 E 2 01 V True False

Physical Chemistry
EquilibriumThe equilibrium constant Ke was found to be 8 4 x108 at 25 C for the following reacti 2X s 3Y aq 2X aq 3Y s What is the cell potential in volt for this reaction Report your answer with 3 places past the decimal point Do not put unit in your answer

Physical Chemistry
EquilibriumThe label on a cylinder of a noble gas became illegible so a student allowed some of the gas to flow into an evacuate bulb with a volume of 300 0 mL until the pressure was 685 torr The mass of the glass bulb increased by 1 45 g its temperature was 27 0 C What is the molar mass of this gas molar gas Which of the Group 8A gases was it Oneon O radon O argon O helium O krypton g mol

Physical Chemistry
EquilibriumIn the reaction Mg s 2HCl aq MgCl2 aq H2 g How does the equilibrium shift if the HCI concentration is increased The equilibrium will initially shift but eventually be uneffected O To the reactants O To the products O No change will occur

Physical Chemistry
EquilibriumUse Le Chatelier s Principle to answer the following N 3 H 2 NH3 heat the H the H the H the H a increases b decreases when the N is decreased when the NH3 is increased when the N is increased when the temperature is decreased C is unchanged

Physical Chemistry
EquilibriumAn aqueous solution consists of 0 12 M Ca and 0 10 M Ba Given that Ksp for BaF2 is 5 x 10 and Ksp for CaF is 3 2 x 10 11 calculate the F needed to separate the ions

Physical Chemistry
EquilibriumThe acid dissociation constants for sulfurous acid H SO3 are K 1 7x102 and Ka 6 4x108 What is the pH of a solution of 0 25 M Na SO3 0 602 3 70 10 3 13 4

Physical Chemistry
EquilibriumO Distilled water O 0 1 M HCl aq O 10 M HCl aq O 0 1 M NaOH aq Question 10 What is the pH of a 0 02 M solution of HCI O 12 3 1 4 1 pts 020

Physical Chemistry
EquilibriumSilver chromate is sparingly soluble in aqueous solutions The Ksp of Ag CrO is 1 12 x 10 2 What is the solubility in mol L of silver chromate in 1 30 M potassium chromate aqueous solution solubility What is the solubility in mol L of silver chromate in 1 30 M silver nitrate aqueous solution solubility What is the solubility in mol L of silver chromate in pure water solubility

Physical Chemistry
Equilibriumhe solubility product for Zn OH is 0 x 10 16 The formation constant for the ydroxo complex Zn OH 2 is 4 6 x 10 7 Part A What is the minimum concentration of OH required to dissolve 1 8x10 2 mol of Zn OH in a liter of solution Express your answer using two significant figures VAE OH min B M

Physical Chemistry
EquilibriumA sample of SO3 is introduced into an evacuated sealed container and heated to 600 K The following equilibrium is established 2SO3 9 2SO2 g O g The total pressure in the system is found to be 3 0 atm and the mole fraction of O2 is 0 12 Part A Find Kp Express your answer using two significant figures Kp VAE www
Physical Chemistry
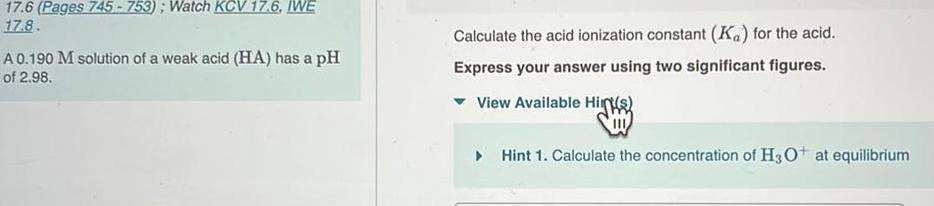
Equilibrium17 6 Pages 745 753 Watch KCV 17 6 IWE 17 8 A 0 190 M solution of a weak acid HA has a pH of 2 98 Calculate the acid ionization constant Ka for the acid Express your answer using two significant figures View Available Hint s Hint 1 Calculate the concentration of H3O at equilibrium

Physical Chemistry
EquilibriumWhat is one set of conjugate acid base pairs in this reaction HCOOH aq H2O I HCOO aq H3O aq A HCOOH and H3O B HCOOH and HCOO C H O and HCOO D H3O and HCOO

Physical Chemistry
Equilibrium10 0 points At a certain temperature the equilibrium constant Ke is 0 154 for the reaction 2 SO2 g O2 g 2SO3 g What concentration of SO3 would be in equilibrium with 0 250 moles of SO2 and 0 758 moles of O2 in a 1 00 liter container at this temperature Note These latter moles are the equilibrium values Answer in units of M

Physical Chemistry
Equilibrium2 When water is saturated with the sparingly soluble CaSO4 three 3 different equilibria exist Write them down a b

Physical Chemistry
EquilibriumA puddle evaporates in the sunshine This is evidence of Choose all the apply An equilibrium system The tendency to minimum enthalpy The tendency to maximum entropy A high activation energy for the reaction

Physical Chemistry
EquilibriumTo calculate the pH at the equivalence point for various types of titrations The equivalence point in an acid base titration is the point at which stoichiometrically equivalent quantities of acid and base have been mixed together At this point the reaction is complete because all analyte has been consumed by titrant On a titration curve the equivalence point is represented by the point of inflection where the curve changes concavity The figure Figure 1 shows the titration of 40 0 mL of 0 100 M HCl with 0 100 M NaOH When 40 0 mL of the NaOH solution is added the acid base neutralization reaction is complete When analyzing titrations involving weak acids or bases consider how the neutralization reaction will impact the pH of the system For example when titrating a weak acid with a strong base at the equivalence point all the base has been used to neutralize the acid forming conjugate weak base Figure Hd 14 12 10 8 6 4 1 of 1 Equivalence point Part B A 80 0 mL volume of 0 25 M HBr is titrated with 0 50 M KOH Calculate the pH after addition of 40 0 mL of KOH at 25 C Express the pH numerically View Available Hint s pH Submit Part C V A O B VAE Review I Constants I Periodic Table Consider the titration of 50 0 mL of 0 20 M NH3 Kb 1 8 x 10 5 with 0 20 M HNO3 Calculate the pH after addition of 50 0 mL of the titrant at 25 C Express the pH numerically View Available Hint s

Physical Chemistry
EquilibriumAdd NaOH Solution 1 00 mL 0 10 mL 0 05 mL Experimental Settings Acid O HCI Volume Base Added 0 00 mL pH 0 3 CH3CO H HCIO Indicator O Methyl Orange NaOH 0 50 M HCI 0 50 M Phenolphthalein Bromothymol blue Retitrate Hd X 14 7 What is the pH at the equivalence point of this titration 25 Volume NaOH aq mL NaOH 12 78 pH 5 88 50

Physical Chemistry
EquilibriumThe reaction of NO g Cl g NOCI g Cl g is found to have an equilibrium constant of 1 1 108 at a particular temperature What does this mean A At equilibrium there is a much higher concentration of reactants than products B At equilibrium there is a much higher concentration of products than reactants C At equilibrium there is an equal concentration of reactants and products D At equilibrium the relative concentrations of reactants and products depends on the initial concentrations
Physical Chemistry
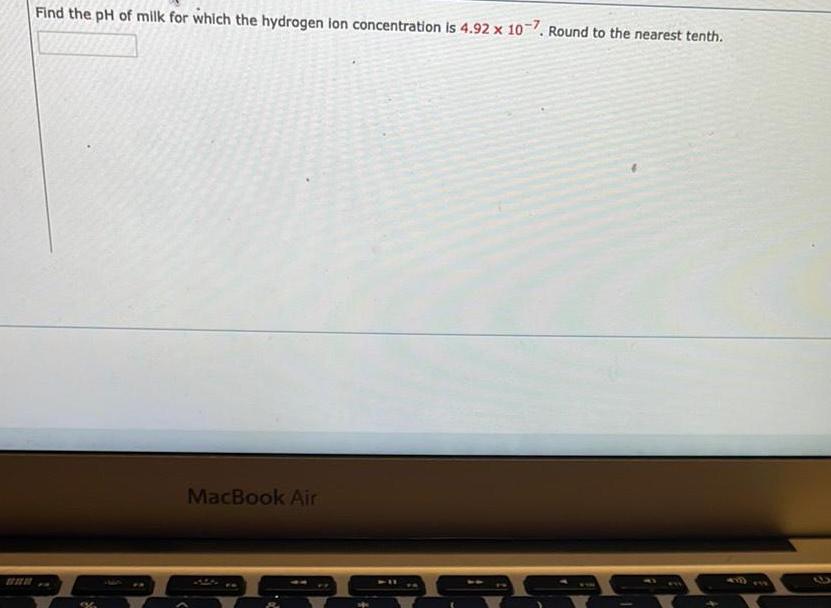
EquilibriumFind the pH of milk for which the hydrogen ion concentration is 4 92 x 10 7 Round to the nearest tenth MacBook Air