Equilibrium Questions and Answers

Physical Chemistry
EquilibriumA chemist dissolves 232 mg of pure potassium hydroxide in enough water to make up 180 mL of solution Calculate the pH of the solution The temperature of the solution is 25 C Round your answer to 3 significant decimal places 11 2 d E

Physical Chemistry
EquilibriumComplete and balance the chemical equation chemical equation Ca H O Name the ionic product of the reaction Spelling counts ionic product I

Physical Chemistry
EquilibriumHow many milliliters of 0 100 M HCIOs are required to neutralize 40 0 mL of 0 195 M KOH

Physical Chemistry
EquilibriumA chemist dissolves 116 mg of pure hydrochloric acid in enough water to make up 270 mL of solution Calculate the pH of the solution Round your answer to 3 significant decimal places 11 O C dh


Physical Chemistry
EquilibriumIn this reaction which of the following factors will NOT change the concentration of CO in the reaction H O g CO g H g CO g a decrease in temperature a decrease in the volume of H O a decrease in pressure an increase in the amount of catalyst


Physical Chemistry
Equilibrium4 Determine the percent ionization of 0 18 M hydrosulfuric acid H S aq The K of H S aq is 8 9 x 10 8

Physical Chemistry
Equilibrium9 What would happen to the equilibrium of this reaction if we remove silver ions Will it shift left increasing the reactants or will it shift right increasing the products Ag aq CI aq AqCl s

Physical Chemistry
EquilibriumThe solubility of silver bromide AgBr in water was determined to be 1 86 x 107 mol L at 20 C The corresponding value of 6 Ks is a b C d 1 36 x 10 3 1 86 x 10 6 3 46 x 10 12 6 43 x 10 18

Physical Chemistry
EquilibriumWhat would happen to the equilibrium of this reaction if we increase the temperature Will it shift left increasing the reactants or will it shift right increasing the products N 3H 2 NH3 Heat

Physical Chemistry
EquilibriumWhat is the pH of the following solutions O Find the pH using this formula pH log H O H O H O 1 0x 10 M H O 1 0x 10M H O 24 x 10 M H O 1 7 x 10 M pH 5 What is the H O of the following solutions 1 66 5 00 10 50 8 00 pH 223E 1000x10 3 00 Sweat Urine H O 11871 56 105 00 Find the H O using this formula H O 10 10 10 50 lo 8 00 WAY pH 1 4 8 4 4 8 5 3 7 3 Acidic Basic or Neutral 6 022 Cerebrospinal fluid Quisic Aride heldie Rombe and sory 2 0 00001 3 16 16 Acidic Basic or Neutral 1 00 x 167 Acidie Acidic Using the following data table list the body fluids in order of INCREASING pH Body Fluid Stomach acid Pancreatic juice Acidic stomach acid 104 Sweat 4 8 Lirine 5 3 Cerebrospinal Fluid 7 3 Pancreatic julte 8 4 Exp 10 v04 Page 7 of 10 7 Which body fluid has the highest hydronium ion concentration H O O 0000016 0 0000050 0 00000000 38 x 10

Physical Chemistry
EquilibriumA 10 mL sample of 0 73M NaOH is mixed with 10mL of 0 48 M H X How much X is in solution after both neutralization reactions are complete H X Ka strong and the Ka 1 5x10 6 for HX

Physical Chemistry
EquilibriumAl2 SO4 3 3Ca OH 2 3Ca SO4 2Al OH 3 How many grams of Ca OH 2 are needed to produce 550 grams of Al OH 32

Physical Chemistry
Equilibrium8 8 Hydrogen chloride can be made from the reaction of chlorine and hydrogen Cl2 g H g 2HCL g For this reaction K 26 x 1033 and AH 44 kcal mol at 25C Is the reaction exothermic or endothermic Are the reactants or the products favored at equilibrium Explain the effects on the equilibrium of Increasing the pressure by decreasing the volume

Physical Chemistry
EquilibriumFor a certain chemical reaction, Qc = 0.2 and Kc= 0.1. Under these conditions, which of the following is true? For full credit, mark all that are true and none that are false. Select one or more:
ΔG < 0
ΔG 0
ΔG° = 0
ΔGO < 0
ΔG° > 0

Physical Chemistry
EquilibriumConsider the following reaction at equilibrium: (ΔH° = +92.4kJ)
2NH3 (g) = N2 (g) + 3H2 (g)
Le Chateliers Principle predicts that the moles of H2 (g) in the reaction
container will increase with
Select one:
O a. addition of some N₂ (g) to the reaction vessel
O b. removal of some N2 (g) from the reaction vessel
O c. a decrease in the total volume of the reaction vessel (constant T)
O d. some removal of NH3 (g) from the reaction vessel
O e. an increase in total pressure by the addition of helium gas (constant T)

Physical Chemistry
EquilibriumWhich of these is NOT a good method for
preparing a buffer? (HA is a weak acid.)
Group of answer choices
Equal volumes of 0.5 M HA and 0.5 M NaA
Equal volumes of 1.0 M HA and 0.5 M
NaOH
Equal volumes of 0.5 M HCl and 0.5 M NaA
Equal volumes of 0.5 M NH3 and 0.5 M
NH4Cl

Physical Chemistry
EquilibriumCobalt ions form complex ions with water and chloride as shown in the reaction. The left side of the reaction is pink, and the right side of the reaction is blue.
Co(H₂O)2 + 4C+ heat <--> CoCl2 + 6H₂O
Which statement about the system at equilibrium is correct?
Adding water to a purple solution will turn it blue.
Adding hydrogen chloride (HCI) to a blue solution will turn it purple.
Heating a blue solution will turn it purple.
Cooling a purple solution will turn it pink.

Physical Chemistry
EquilibriumA solution has a hydrogen ion concentration of 1.32 x 10-³ mol/L. Determine the
pH of the solution.
a) 2.88
b) -0.12
c) 0.12
d) -2.88


Physical Chemistry
EquilibriumWhen an acid, HA, is titrated with 0.1 M NaOH, the pH at the half equivalence point of the titration is 4.5. What is the Ka of the acid?
Multiple Choice
32x10-5
3.2x10-10
1.8 x 10-3

Physical Chemistry
EquilibriumStudy this chemical reaction:
FeSO4(aq) + Mg(s) Fe(s)+MgSO4(aq)
Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
oxidation:
reduction:
![The solubility of strontium fluoride, SrF2, in moles per liter, can be expressed in terms of the resulting ion concentrations. Which relationship is correct?
solubility=[2Sr2+]
solubility=[Sr2+]
solubility= 2[Sr2+]
solubility=[F-]
solubility= 2[F-]](https://media.kunduz.com/media/sug-question/raw/74332484-1660064302.108218.jpeg?w=256)
Physical Chemistry
EquilibriumThe solubility of strontium fluoride, SrF2, in moles per liter, can be expressed in terms of the resulting ion concentrations. Which relationship is correct?
solubility=[2Sr2+]
solubility=[Sr2+]
solubility= 2[Sr2+]
solubility=[F-]
solubility= 2[F-]

Physical Chemistry
EquilibriumA chemist must prepare 600.0 mL of sodium hydroxide solution with a pH of 13.70 at 25 °C.
She will do this in three steps:
Fill a 600.0 mL volumetric flask about halfway with distilled water.
Weigh out a small amount of solid sodium hydroxide and add it to the flask.
Fill the flask to the mark with distilled water.
Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits.

Physical Chemistry
EquilibriumA solution is prepared at 25 °C that is initially 0.49 M in benzoic acid (HCH,CO₂), a weak acid with K = 6.3 x 10, and 0.25M in potassium benzoate (KC6H5CO₂). Calculate the pH of the solution. Round your answer to 2 decimal places.

Physical Chemistry
EquilibriumHow does adding an inhibitor affect the rate of a reaction?
It does not affect the rate of a reaction
suppresses the production of activated complexes
increases activation energy
decreases activation energy

Physical Chemistry
EquilibriumAmmonia is a convenient buffer system in the slightly basic range.
(a) What is the pH of a buffer solution containing 39 g of NH4Cl dissolved in 1.00 L of 0.940 M NH3?
pH =
(b) How many moles of acid are required to change the pH of this solution by 0.01 pH units?
mol
(c) Suppose 5.6 mL of 10.5 M HCI solution is added to 230 mL of the solution of Part (a). Calculate the new pH.
pH=

Physical Chemistry
EquilibriumDetermine the pH during the titration of 69.3 mL of 0.392 M benzoic acid (Ka = 6.3x10-5) by
0.392 M NaOH at the following points.
(a) Before the addition of any NaOH
(b) After the addition of 15.0 mL of NaOH
(c) At the half-equivalence point (the titration midpoint)
(d) At the equivalence point
(e) After the addition of 104 mL of NaOH

Physical Chemistry
EquilibriumWrite the net ionic equation for the neutralization of nitric acid by sodium hydroxide in aqueous solution.
H+(aq) + OH¨(aq) → H₂O(l)
H+(aq)+NO3(aq) +OH-(aq)→ NO3(aq)+ H₂O(l)
HNO3(aq) +Na+(aq)+ OH-(aq)→ Na+(aq) +NO3(aq) +H₂O(l)
HNO3(aq) +OH-(aq)→ NO3-(aq) + H₂O(l)

Physical Chemistry
EquilibriumIf 20.0 mL of HNO3(aq) is used to neutralized
50.00 mL of 0.200 mol/L NaOH,
a) What is the balanced chemical equation.
b) Find the molar concentration of the HNO3?
c) What is the pH at the end of the
titration?
![The equilibrium constant for the following
reaction:
Ca(HCO3)2(s) <--> CaO(s) + 2 CO₂(g) + H₂O(g)
would be:
O K = [CaO][CO₂]²[H₂O] / [Ca(HCO3)2]
O K = [CO₂]²[H₂O]
OK = [CO₂]²[H₂O] / [Ca(HCO3)2]
O K = [Ca(HCO3)2] / [CaO][CO₂]²[H₂O]](https://media.kunduz.com/media/sug-question/raw/76287939-1659793035.9207506.jpeg?w=256)
Physical Chemistry
EquilibriumThe equilibrium constant for the following
reaction:
Ca(HCO3)2(s) <--> CaO(s) + 2 CO₂(g) + H₂O(g)
would be:
O K = [CaO][CO₂]²[H₂O] / [Ca(HCO3)2]
O K = [CO₂]²[H₂O]
OK = [CO₂]²[H₂O] / [Ca(HCO3)2]
O K = [Ca(HCO3)2] / [CaO][CO₂]²[H₂O]

Physical Chemistry
EquilibriumGiven the following reaction:
XBO3(s) <--> XO(s) + BO₂(g)
If the concentration of BO2 at equilibrium was
2.0 M, what is the equilibrium constant (K)?
O 2.0 M
O 0.50 M
O 1.0 M
O Not enough information is given

Physical Chemistry
EquilibriumA chemistry graduate student is given 250. mL of a 0.40M acetic acid (HCH3CO₂) solution. Acetic acid is a weak acid with K=1.8 x 10³. What mass of
KCH3CO2 should the student dissolve in the HCH3CO2 solution to turn it into a buffer with pH = 4.30?
C
You may assume that the volume of the solution doesn't change when the KCH3CO₂ is dissolved in it. Be sure your answer has a unit symbol, and round it to
2 significant digits.
0
X

Physical Chemistry
EquilibriumIn Beaker A, an antacid tablet is added to 50 mL of 20°C water. In Beaker B, an
antacid tablet is added 50 mL of 70°C water. In which beaker will the reaction
occur faster?
B
Oboth will occur at the same rate
OA

Physical Chemistry
EquilibriumA puddle evaporates in the sunshine. This is evidence of...
Choose all the apply
An equilibrium system
The tendency to minimum enthalpy
The tendency to maximum entropy
A high activation energy for the reaction

Physical Chemistry
EquilibriumConsider the following system at equilibrium where AH = 108 kJ, and Ke 1.29x102, at 600 K:
=
cocl₂(g) co(g) + Cl₂(9)
If the TEMPERATURE on the equilibrium system is suddenly increased:
The value of Ke
The value of Qc
The reaction must:
A. Increases
B. Decreases
C. Remains the same
A. Is greater than Ke
B. Is equal to Ke
C. Is less than Ke
A. Run in the forward direction to restablish equilibrium.
B. Run in the reverse direction to restablish equilibrium.
C. Remain the same. Already at equilibrium.
The concentration of Cl₂ will:
A. Increase.
B. Decrease.
C. Remain the same.

Physical Chemistry
EquilibriumIn lab, students are given 100.0 mL of 1M hydrochloric acid, HCI, to make a product.
Which of the following steps should the students take in order to increase the rate of
reaction between hydrochloric acid and sugar?
Increase the volume of hydrochloric acid to 200.0 mL because a greater amount
of reactant results in a faster reaction rate.
Increase the concentration of hydrochloric acid to 2M because
greater concentrations result in more collisions between the reactants.
Decrease the volume of hydrochloric acid to 50.0 mL because a lower amount of
reactant results in a faster reaction rate.
Decrease the concentration of hydrochloric acid to 0.5M because
lower concentrations result in more collisions between the reactants.

Physical Chemistry
EquilibriumStudents are trying to calculate the heat of reaction for the following target reaction.
A + 3B 3C + 2D
They find the heat of reactions for the two reactions as shown.
A + 2B C + D
AH = 20 kJ
B → 2C + D
AH-45 kJ
When the students used Hess's Law correctly, what is the heat of reaction for the
target reaction?
+65 kJ
O-25 kJ
+25 kJ
O-65 kJ

Physical Chemistry
EquilibriumUnder certain conditions, the equilibrium constant K, for the decomposition of
PCI(g) into PCls(g) and Cl₂(g) is 0.0211. What are the equilibrium concentrations of
PC15, PCs, and Cl₂ in a mixture that initially contained only PCI, at a concentration
of 1.00 M?

Physical Chemistry
EquilibriumIf Qsp > Ksp (Qsp is greater than Ksp)
Solution is supersaturated; no precipitation will form
Solution is saturated; precipitation will form
Solution is unsaturated; no precipitate will form
Solution is saturated; no precipitation will form
Solution is supersaturated; precipitation will form
Solution is unsaturated; precipitate will form

Physical Chemistry
EquilibriumThe solubility product Ksp for Ag3PO4 is 2.7 x 10-18. What is the solubility of silver phosphate in a solution which also contains 0.22 moles of silver nitrate per liter?
Report your answer in scientific notations with ONE place past the decimal point.
Use this format: 1.2*10^-3
Do not add units in your answer.
Type your answer...

Physical Chemistry
EquilibriumTrimethylamine, (CH3)3N, is a weak base with pKs = 4.19 at 25°C. A 25.00 mL sample of an aqueous solution
of trimethylamine is titrated with 0.125 MHCI. The neutralization reaction is as follows:
(CH3)3N(aq) + HCl(aq) → (CH3)3NH+(aq) + Cl-(aq)
The equivalence point of the titration is reached after the addition of 20.38 mL of HCl solution.
(a) What is the concentration of the trimethylamine solution?
(b) What is the pH of the solution at the equivalence point?

Physical Chemistry
EquilibriumTrimethylamine, (CH3)3N, is a weak base with pKb = 4.19 at 25°C. A 25.00 mL sample of an aqueous solution
of trimethylamine is titrated with 0.125 MHCI. The neutralization reaction is as follows:
(CH3)3N(aq) + HCl(aq) → (CH3)5NH- (aq) + Cl(aq)
The equivalence point of the titration is reached after the addition of 20.38 mL of HCl solution.
(a) What is the concentration of the trimethylamine solution?
(b) What is the pH of the solution at the equivalence point?

Physical Chemistry
EquilibriumBefore the bottle is squeezed, the system is at equilibrium. After the bottle has been squeezed, the system eventually returns to equilibrium. Is the concentration of CO₂ the same in both equilibrium situations (before and after squeezing the bottle)? What about the equilibrium constant?

Physical Chemistry
EquilibriumGold(III) chloride, AuCl3, has Kp = 3.2 x 10-25. Calculate the molar solubility of gold (III) chloride in pure water and in various aqueous solutions.
Calculate the molar solubility of gold(III) chloride in pure water.
Enter your response in scientific notation, e.g. enter 2E3 for 2000.
M
Calculate the molar solubility of gold(III) chloride in 0.010 M HCI solution.Enter your response in scientific notation, e.g. enter 2E3 for 2000.

Physical Chemistry
EquilibriumA chemist needed an aqueous buffer with a pH of 3.90.
Part a
Would formic acid and its salt, sodium formate, make a good pair for this purpose?
Formic acid and its salt, sodium formate would make a good pair for this purpose.
Formic acid and its salt, sodium formate would not make a good pair for this purpose.
What mole ratio of the acid, HCHO2, to the anion of this salt, CHO₂-, is needed?
mol HCHO2 initial/mol CHO₂- initial=
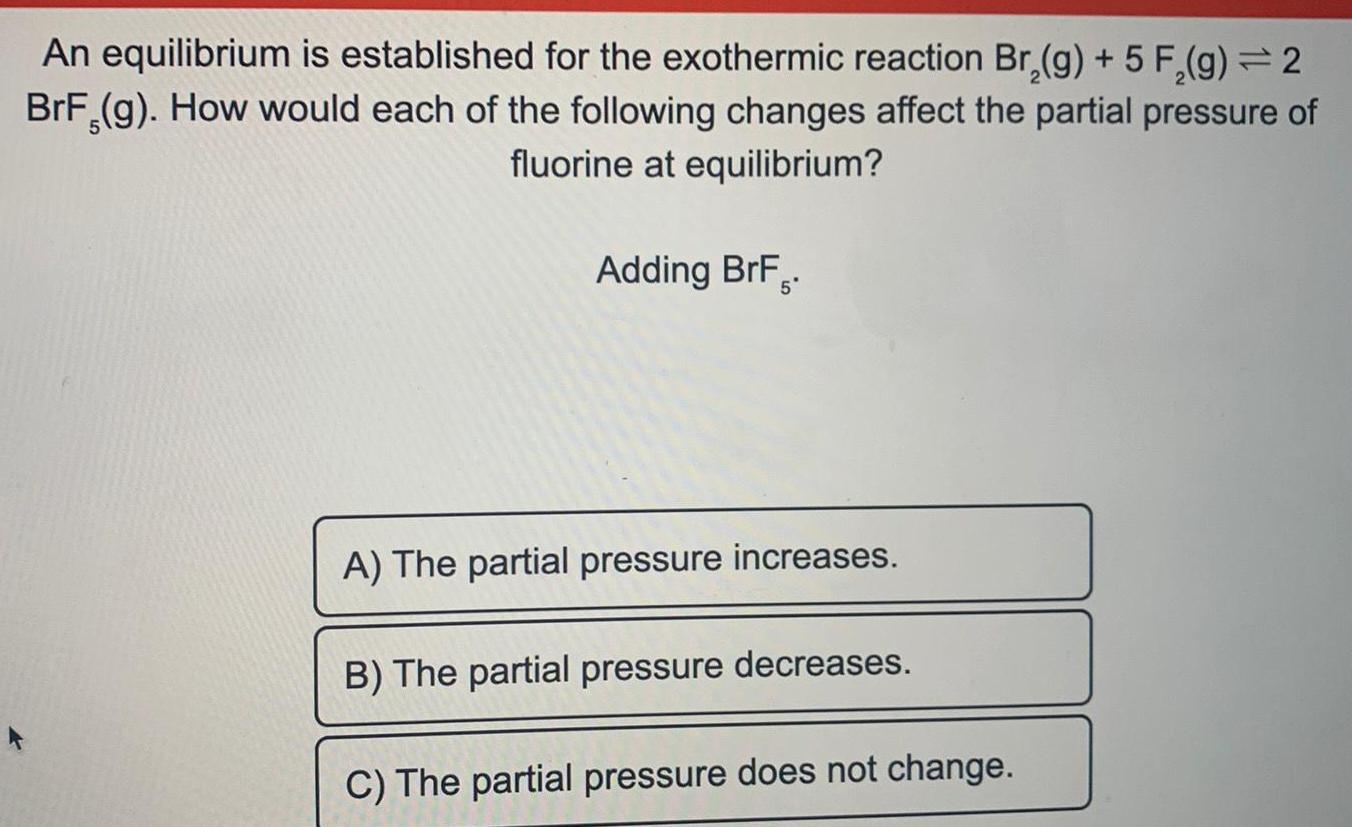
Physical Chemistry
EquilibriumAn equilibrium is established for the exothermic reaction Br₂(g) + 5 F₂(g) <->2BrF3 (g). How would each of the following changes affect the partial pressure of fluorine at equilibrium?
Adding BrF.
A) The partial pressure increases.
B) The partial pressure decreases.
C) The partial pressure does not change.

Physical Chemistry
EquilibriumThe Ksp value for Al(OH)3 is 3.0 x 10-34.
Part 1
Calculate the pOH in an Al(OH)3 solution that is slightly basic with a pH of 9.50.
pOH = M

Physical Chemistry
EquilibriumWhen butter turns rancid, its foul odor is mostly that of butanoic acid, HC4C7O2, a weak monoprotic acid similar to acetic acid in
structure. In a 0.0100 M solution of butanoic acid at 20 °C, the acid is 4.0% ionized.
Calculate the K₂ of butanoic acid at this temperature.
Ka=
Calculate the pK, of butanoic acid at this temperature.
pKa =