Gaseous and liquid states Questions and Answers

Physical Chemistry
Gaseous and liquid statesTwo flasks of capacity 1 L and 2 L contain gases A and B respectively at same temperature If density of A is 2 5 g L and that of B is 5 g L and the molar mass of A is twice of that of B then the ratio of pressure exerted by gases A B is NCERT Pg 146 1 1 2 2 1 4 1 2

Physical Chemistry
Gaseous and liquid statesIf the slope of Z versus p curve is constant slope atm at a partic P 492 6 300 K and at very high pressure then diameter of the molecules B 50 C 2 5A D 1 25 temperature A 7 5A

Physical Chemistry
Gaseous and liquid states1 g of a weak monobasic acid MW 100 when dissolved in 100 g of liquid A MW 100 increases the boiling point from 80 C to 81 C at 760 mm of Hg The vapour pressure of liquid A at 90 C is 850 mm of Hg Degree of dissociation of the monobasic acid is 0 189 at 90 C Enthalpy of neutralization of a strong acid with strong base 57 3 kJ equivalent

Physical Chemistry
Gaseous and liquid states47 A gas is collected in flask as shown below a gas 50 mmHg The pressure exerted by the gas when the atmospheric pressure is 0 9 atm is abc mmHg The value of a b c is e g if pressure is 123 mmHg then answer will be 1 2 3 6

Physical Chemistry
Gaseous and liquid states19 The value of ebullioscopic constant depends upon 1 AHsoution 3 Nature of solute 2 Nature of solvent 4 Freezing point of solution topic with 1 solution of a non volatile non electrolyte substance Ther

Physical Chemistry
Gaseous and liquid states14 Pressure exerted by a perfect gas is equal to 1 Mean kinetic energy per unit volume 2 Half of the mean kinetic energy per unit volume 3 Two thirds of mean kinetic energy per unit volume 4 One third of mean kinetic energy per unit volume
Physical Chemistry
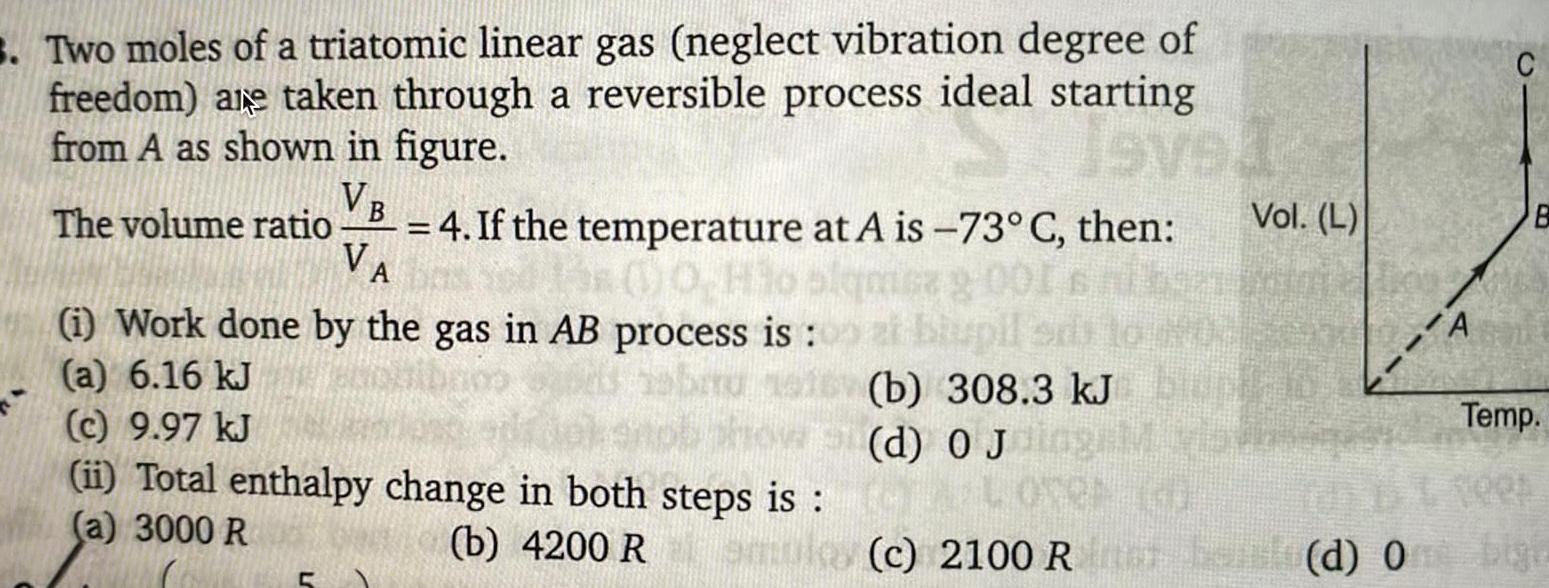
Gaseous and liquid states3 Two moles of a triatomic linear gas neglect vibration degree of freedom are taken through a reversible process ideal starting from A as shown in figure Seved VB The volume ratio sa 9 00 VA 4 If the temperature at A is 73 C then 0 Ho i Work done by the gas in AB process is a 6 16 kJ c 9 97 kJ ii Total enthalpy change in both steps is a 3000 R Vol L al biupil b 308 3 kJ BUT d 0 Jolag vis CA LOVER b 4200 Romulos c 2100 Ron A B Temp Tees belo d One bigi

Physical Chemistry
Gaseous and liquid statesWhich of the following is correct about Contact process for the manufacture of sulphuric acid a V 05 is used for catalytic oxidation of SO to SO3 b SO2 is produced which is absorbed in water c SO3 is directly absorbed in water SO3 is absorbed in concentrated sulphuric acid d Only One Correct Answer

Physical Chemistry
Gaseous and liquid statesI know that this is something we practically observe that all gases stay diffused throughout the s pace despite their molecular weight but I am not able to understand why this is so because if u see then in cold air and warm air cold air being denser settles at bottom whereas warm air remain s on top then why isn t the same principle applicable for molecular masses i e one with higher molecular mass comes to bottom and the lighter one stays at top I read an available answer on it utor and the teacher says that molecular mass doesn t show heaviness of a gas But if this was so then why do we say that lighter gases diffuse out faster out of an orifice than heavier ones The mixture of three gases X Y and Z is enclosed in a closed vessel at constant temperature Molecular weight of X is the highest and that of Y is the least When equilibrium is established the 1 Gas X will be more at bottom 2 Gas Y will be more at top 3 Gas X Y Z are homogeneously present 4 Gas Y will be more at bottom ns

Physical Chemistry
Gaseous and liquid statesA scuba diver ascends too quickly and develops the bends A nitrogen bubble has formed in the patient s elbow At a depth of 50 ft where the pressure is 2 51 atm the bubble had a volume of 0 018 mL Assuming a constant temperature and number of moles of nitrogen in the bubble what volume did the bubble increase to at the surface where the pressure is 1 00 atm bubble volume at surface The scuba diver is placed into a hyperbaric oxygen chamber where the pressure is 3 98 atm What is the volume of this same nitrogen bubble while the patient is in the hyperbaric chamber bubble volume in chamber mL ml

Physical Chemistry
Gaseous and liquid statesConsider the following manometer What is the gas pressure if the air pressure is equal to 760 mml 20 cm O 100 mmHg 860 mmHg 960 mmHg 660 mmHg Atmospheric pressure F 10 cm Mercury

Physical Chemistry
Gaseous and liquid statesA mixture of chlorobenzene and water immiscible is heated and boiled at 1 atm Assuming that the vapors behave like the ideal gas determine at what temperature the Mix and calculate the molar composition of the distillate No 1 2 Nombre Antoine p bar T K A B C Agua 5 40221 1838 67 31 737 Clorobenceno 4 11083 1435 675 55 124 A B and C are the Antoine ecuation constants for Water 1 and chlorobenzene 2 with coccure in ar and Tamn in

Physical Chemistry
Gaseous and liquid statesDuring Test 4 it was determined that the pressure for 100 blue heavy particles and 100 red ligh particles is less than the pressure for 200 blue heavy particles Therefore for an ideal gas the total pressure of a system is independent from particle size Answer 1 less than Answer 2

Physical Chemistry
Gaseous and liquid states9 0 1 mole of N 204 g was sealed in a tube under one atmospheric conditions at 25 C Calculate the number of moles of NO 2 g present if the equilibrium N 04 g 2NO g Kp 0 14 is reached after some time P a 1 8 x 102 b 2 8 x 10 0 034 d 2 8 x 10 2

Physical Chemistry
Gaseous and liquid statesA sample contains two different ionic species at different concentrations The two ions can be distinguished in polarography by O Half wave potentials Polarography is confined to O solutions containing single type of ions only By addition of one ion such that their concentrations are equal O Diffusion currents

Physical Chemistry
Gaseous and liquid statesFor the estimation of nitrogen 1 4 g of an organic compound was digested by Kjeldahl method and the M evolved ammonia was absorbed in 60 mL of sulphuric acid The unreacted acid required 20 mL of 10 M 10 sodium hydroxide for complete neutralizaton The percentage of nitrogen in the compound is JEE Main online 2014 A 3 B 5 C 6 D 10

Physical Chemistry
Gaseous and liquid statesThe densities of solid and liquid silver MW 107 9 g mol are 11 7 g mL and 10 5 g ml respectively The normal melting point of silver is 962 C The enthalpy of fus of silver is 12 0 kJ mol What is the melting point in C of silver under an applied pressure of 500 atm

Physical Chemistry
Gaseous and liquid states1 12 pts Describe how you would measure the density at room temperature of a pure substance composed only of this molecule You will need to figure out if this substance is a solid liquid or gas at room temperature and make your plan according to this Be creative and assume you have access to whatever glassware equipment and instruments you need a Describe your stepwise procedure here Include details about the kind of glassware and equipment you are using for each step Make sure you include any calculations you would need to perform on your measurement s to determine the density b Choose one measurement e g mass volume length etc you described in a Describe one way

Physical Chemistry
Gaseous and liquid states1 A steel tank contains air at a pressure of 15 bar at 20 C The tank is provided with safty valve which can withstand a pressure of 35 bar Calculate the temperature to which tank can be safely heated

Physical Chemistry
Gaseous and liquid states5 1 g of NH3 and 3 g of NO are mixed and kept in a container of 1 L at 27 C The total pressure exerted by gases is x Then the value of 10 x in atm is Assume gases are non reacting and ideal Take R 0 08 atm L K mol Answer HII 0 1 2 3 4 5 6 7 8 9

Physical Chemistry
Gaseous and liquid statesCalculate the pressure exerted by 1023 gas molecules each mass 10 g in litre The rms velocity is 105 cm sec L A 3 33 x 10 dyne cm C 3 33 x 10 dyne cm What must be the temperature A 3614 8 K B 3 33 x 105 dyne cm D 3 33 x 10 dyne cm B 2214 8 K P What is the total kinetic energy in cal of these particles A 1175 0 Cal B 1195 0 Cal C 1155 0 Cal D 1185 0 Cal 3 PV 3x3 33x107x10 D 2414 8 K 2 P 1 C 1214 8 K I mnc Ixlx 25 1000 T K 6 022 11 8 314x TX107

Physical Chemistry
Gaseous and liquid statesThe compression factor compressibility factor for one mole of a vander Waals gas at 0 C and 100 atmosphere pressure is found to be 0 5 Assuming that the volume of a gas molecule is negligible calculate the vander waals constant a A 1 2544 atm L2 mol B C 125 44 atm L2 mol 12 544 atm L2 mol 2 2

Physical Chemistry
Gaseous and liquid states1 NH 2 C 3 SO 4 CO 11 1 mol CO ocupies 0 4 lit at 27 C and 40 atm calculate the compressibility factor 1 0 65 2 0 85 3 0 15 D 1 65

Physical Chemistry
Gaseous and liquid statesAir contains 79 N and 21 O by volume If th total pressure is 750 mm of Hg then the part pressure of O is 1 157 2 mm of Hg 2 175 5 mm of Hg 3 315 0 mm of Hg 4 None of these

Physical Chemistry
Gaseous and liquid states3 0 85 litre 12 One litre of CO is passed through red hot coke The volume becomes 1 4 litres at same temperature and pressure The composition of products is co C 200 1 0 8 litre of CO and 0 6 litre of CO Pq no 2 0 7 litre of CO and 0 7 litre of CO 63 3 4 41 4 2 0 6 litre of CO and 0 8 litre of CO 0 4 litre of CO and 1 0 litre of CO 3
Physical Chemistry
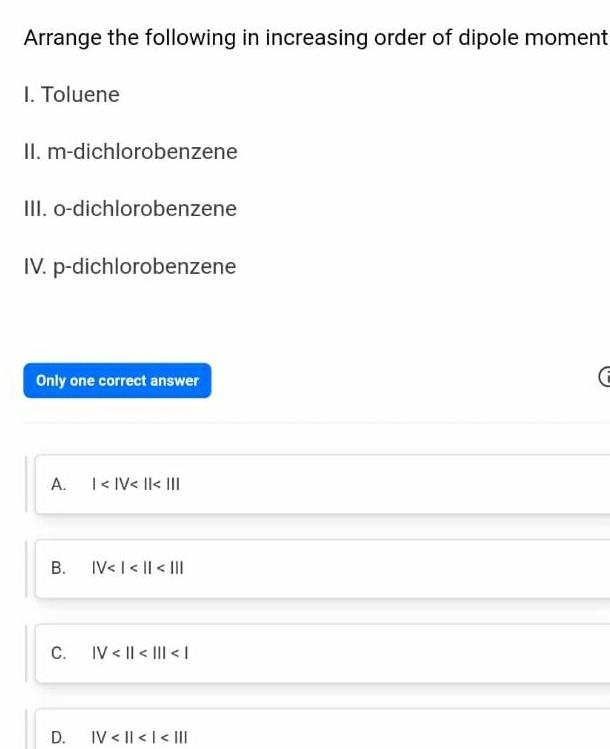
Gaseous and liquid statesArrange the following in increasing order of dipole moment I Toluene II m dichlorobenzene III o dichlorobenzene IV p dichlorobenzene Only one correct answer A B C D IV IV IV IV

Physical Chemistry
Gaseous and liquid statesNEET UG 2019 A gas at 350 K and 15 bar has molar volume 20 percent smaller than that for an ideal gas under the same conditions The correct option about the gas and its compressibility factor Z is 1 2 1 and attractive forces are dominant 2 Z 1 and repulsive forces are dominant 3 Z 1 and attractive forces are dominant

Physical Chemistry
Gaseous and liquid states21 For the following equilibrium N O 2NO K is found to be equal to K This is attained when 1 T 1 K 2 T 12 18 K 4 T 273 K 3 T 27 3 K 26

Physical Chemistry
Gaseous and liquid stateszal 29 The values of van der Waal s constant a for the gases O N NH and CH are 1 360 1 390 4 170 and 2 253 L atm mol2 respectively The gas which can most easily be liquefied is 1 0 2 N 3 NH 4 CH RG0031

Physical Chemistry
Gaseous and liquid states302 15 Pre Medical Chemistry 50 ml of a gas A diffuse through a membrane in 22 the same time as for the diffusion of 40 ml of a gas B under identical pressure and temperature conditions If the Molecular weight of A 64 that of B would be JOL OF Vache 1 00

Physical Chemistry
Gaseous and liquid states7 50 ml of hydrogen diffuses through a small hole from vessel in 20 minutes time Time taken for 40 ml of oxygen to diffuse out under similar conditions will be 1 12 min 2 64 min 3 8 min 4 32 min 24

Physical Chemistry
Gaseous and liquid states4 a c b d Q 119 The temperature at which the most probable Q 119 speed of SO gas is equal to the most probable speed of O gas at 27 C is 1 327 C 3 723 C 2 273 C 4 373 C 4 a c b d f o ft tu Caff af en RIEK 1 327 C 3 723 C 2 273 C 4 373 C

Physical Chemistry
Gaseous and liquid states11 The relative rate of diffusion of a gas Mol wt 98 as compared to hydrogen will be 2 1 5 3 1 4 2022 4 1 IG0011

Physical Chemistry
Gaseous and liquid stateshow much volume be added to get pk of the acid 5 mL 4 100 mL ALLEN 117 K for HCN is 5 x 10 10 at 25 C For maintaining a constant pH of 9 the volume of 5M KCN solution required to be added to 10mL of 2M HCN solution is 1 4 mL 2 7 95 mL

Physical Chemistry
Gaseous and liquid statesIEO143 135 OH 121 A basic buffer will obey the equation POH PK 1 only under condition Conjugate acid base 1 10 136 2 Conjugate acid base 3 Conjugate acid base 10 1 4 None of these 202

Physical Chemistry
Gaseous and liquid statesA sample of xenon gas at a pressure of 1 13 atm and a temperature of 290 C occupies a volume of 700 mL If the gas is cooled at constant pressure until its volume is 576 ml the temperature of the gas sample will be

Physical Chemistry
Gaseous and liquid statesEqual weights of ethane and hydrogen are mixed in an empty container at 25 C The fraction of the total pressure exerted by hydrogen is 1 1 2 2 1 1 3 1 16

Physical Chemistry
Gaseous and liquid statesUnder what conditions will a pure sample of an ideal gas not only exhibit a pressure of 1 atm but also a concentration of 1 mol L R 0 082 L atm mol deg 1 At STP 2 When V 22 4 L 3 When T 12 K 4 Impossible under any conditions

Physical Chemistry
Gaseous and liquid statesCE0073 um tate CE0074 68 The equation a 1 A Where D Theoretical vapour density d Observed vapour density A 4 A D d n 1 d nB nC 2 3 20201 2n 3 B is correctly matched for B C

Physical Chemistry
Gaseous and liquid states11 per hour 1 71 50 A 3 142 96 A 2 35 70 A 4 285 93 A A solution is prepared by mixing 8 5 g of Q 11 CH Cl and 11 95 g of CHCl3 If vapour pressure of CH Cl and CHCl at 298 K are 415 and 200 mm Hg respectively the mole fraction of CHCI in vapour form is Molar mass of Cl 35 5 g mol 1 0 162 3 0 325 2 0 675 4 0 486 CRICT mier 1 71 50 A 3 142 96 A CH Cl 8 5 CHCI 11 95 feller va facra aur fou via iuf 298 K 415 CH Cl 3 CHC aft 200 mm Hg 2 35 70 A 4 285 93 A CHC 35 5 g mol 1 0 162 3 0 325 2 0 675 4 0 486

Physical Chemistry
Gaseous and liquid states59 The solubility product of three sparingly soluble salts are given below No Formula Solubility product 1 4 0 x 10 20 2 3 2 x 10 14 3 2 7 x 10 7 The correct order of decreasing molar solublity is 1 1 2 3 2 2 1 3 3 3 2 1 4 2 3 1 IE0081 PQ PQ PQ sion

Physical Chemistry
Gaseous and liquid statesThe number of atoms contained in 11 2 L of SO at N T P are 1 3 2 x 6 02 x 1023 2 2 x 6 02 x 1023 3 6 02 x 1023 4 4 6 02 1023

Physical Chemistry
Gaseous and liquid states124 41 Pre Medical Chemistry 4 mole of PC are heated at constant temperature 47 I in closed container If degree of dissociation for PCI is 0 5 then calculate total number of moles at 2 6 3 3 4 4 equilibrium 1 4 5 C C

Physical Chemistry
Gaseous and liquid statesIn a 20 litre vessel initially 1 1 mole CO H O CO is present then for the equilibrium of CO H O 1 H more than 1 mole 2 CO H O H less than 1 mole 3 CO H O both more than 1 mole 4 All of these CO H following is true

Physical Chemistry
Gaseous and liquid statesThe volume of gases NH3 CO and H adsorbed by one gram of charcoal at 300 K are in order of 1 H CO NH3 NHg Hy CO2 NH3 CO2 H CO2 NH3 H2 Correct Answer 3 Status unattempted

Physical Chemistry
Gaseous and liquid statesUSE CODE BAHUBALI FOR 10 OFF ON PLUS ICONIC SUBSCRIPTION Four one litre flasks are separately filled with the gases hydrogen helium oxygen and ozone at the same room temperature and pressure The ratio of total number of atoms of these gases present in the different flasks would be 1 1 1 1 1 2 1 2 2 3 3 2 1 2 3 4 3 2 2 1

Physical Chemistry
Gaseous and liquid states4 11 2 L Hat STP is produced for every mole HCL consumed The vapour density of gas A is four times that of B If molecular mass of B is M then molecular mass of A is 1 M 2 4M 3 M 4 4 2M

Physical Chemistry
Gaseous and liquid statesIn the reaction 2AL 6HCL 2AP 6C 3H g 1 6L HCL is consumed for every 3L H g produced 2 33 6 L H is produced regardless of temperature and pressure for every mole Al that reacts 3 67 2 L H at STP is produced for every mole Al that reacts 4 11 2 L Hat STP is produced for every mole HCL consumed

Physical Chemistry
Gaseous and liquid states21 Boiling point of 0 01 M AB which is 10 dissociated in aqueous medium 0 52 as 476 A and B 1 273 006 K 3 0 006 K 2 373 006 K 4 272 006

Physical Chemistry
Gaseous and liquid statesA circle of radius R is drawn in a uniform electri field E as shown in the fig V V Vc and V an respectively the potentials of points A B C and D on the circle then 1 VA Vc V Vp 2 V Vc VB VD 3 V VC V VD 4 V V V VD D F6005