General Questions and Answers

Physical Chemistry
GeneralWhat is the total volume of gases produced when 3 0 L of nitrogen trichloride NCIS decomposes into chlorine and nitrogen at a constant pressure and A 3 0 L B 4 0 L C 6 0 L D 8 0 L temperature 2NCI g N g 3Cl g

Physical Chemistry
General1 2 points The highest possible percent yield in a chemical reaction is O 95 100 99 O 90

Physical Chemistry
General3 If 23 5 grams of silver nitrate are added to 16 0 grams of sulfuric acid how many grams of silver sulfate will be produced

Physical Chemistry
GeneralA reported barometric pressure is 5 33x104 pascals What is this pressure in atmospheres Conversion factors 1 atm 760 mm Hg 760 torr 101325 pascals 29 92 in Hg atm

Physical Chemistry
GeneralOf the following indicate which are aldoses OD Fructose Glyceraldehyde OL Glucose OD Sorbose D Threose 45

Physical Chemistry
General5 Write the chemical names for the following compounds a Ba NO3 2 b NaClO3 how way wade of gay lagoitibba sou vel ahops guiwallot 1000 c NH4ClO2 d Mn NO2 2

Physical Chemistry
GeneralIndicate which of the following is a saturated fatty acid Select all that apply stearic acid Olinolenic acid DCH CH2 4 CH CHCH2 4 CH2 2COOH CH3 CH2 14 COOH none of the above

Physical Chemistry
GeneralWhich of the following incorrectly describes a chemical system Closed systems permit the transfer of energy O Open systems permit the transfer of matter and energy O Isolated systems do not permit the transfer of matter or energy O Closed systems do not permit the transfer of matter or energy

Physical Chemistry
General6 When 45 0 grams of sodium hydroxide are mixed with 56 0 grams of silver nitrate how many grams of silver hydroxide are produced

Physical Chemistry
GeneralCalculate the concentration of an HCI solution if 100 0 mL of the HCI required 33 00 mL of 0 2000 M Mg OH 2 to reach the titration endpoint Mg OH 2 2 HCI 2 H O MgCl There is enough information to calculate the moles of base but not the moles of acid LX mol Mg OH 3 mol HCl mol L mol HCI mol Mg OH 2 mol Mg OH 3 mol HCI W

Physical Chemistry
Generalsample will have the greatest mass A 1 mol of SO B 1 mol of SO3 C 2 mol of SO D 2 mol of SO3

Physical Chemistry
Generalgovernment issues warnings and evacuations concerning catastrophic weather events it is exercising its O police power O force in action O foresight O weather forecasting abilit

Physical Chemistry
GeneralWhich of the following statements about bond energy calculations to find AHrxn is true O The result is similar to formation reaction calculations regardless of the state of the reactants and products The result will be similar to formation reaction calculations if all the reactants and products are gases O The result will be similar to formation reaction calculations if all the reactants and products are liquids O The result will be similar to formation reaction calculations if all the reactants and products are solids

Physical Chemistry
General47 2 points A box of Barbara s Organic Grain Shop cereal contains 15 servings and each serving contains 120 mg of sodium How many boxes of cereal would contain 12 moles of sodium 196 2588 173 19 6 Previous Next

Physical Chemistry
Generalacid Base REVIEW When an acid is dissolved in water it will A release H ions into the water B release H ions into the water E not release ions into the water release OH ions into the water D 2 Sodium hydroxide NaOH is a strong base because it A Does not dissolve in water B Reacts to form salt crystals in water C Easily releases hydroxide ions D Does not conduct an electric current 3 Ammonia NH3 is a base What best describes ammonia when dissolved in water A ammonia forms a neutral solution B ammonia forms a buffered solution C ammonia acts as a hydrogen ion donor ammonia acts as a hydrogen ion acceptor D 4 Which of the following is a true statement A strong and weak acids dissociate equally B strong acids dissociate fully in solution while weak acids dissociate partially C strong acids are weak electrolytes D strong acids dissociate partially in solution while weak acids dissociate fully 5 Sulfurous acid H SO forms from SO and water in the atmosphere to produce acid rain Since it acts as an 9 Which of these is the best conductor of electricity A Distilled water C 1M NaCl D Milk B tap water 10 Which property do most acids share A B Slippery Sour taste C Faint color D Bitter taste 11 Which of these is a property of most bases A They dissolve metals B They break down oil and dirt C They dissolve carbonate compounds D They react to proteins 12 HCI fully dissociates to form hydrogen ions What type of substance is HCI A weak acid B weak base C strong base D strong acid 13 Lithium Hydroxide LiOH is a strong base because i A does not dissolve in water B easily releases hydroxide ions C D reacts to form a strong crystal lattice in water does not conduct an electric current 14 What evidence best indicates that ethanol C H5OH is a weak acid

Physical Chemistry
GeneralCurrent Attempt in Progress At 14 0 C and a pressure of 773 torr a gas was found to have a density of 2 33 g L Calculate its molar mass g mol

Physical Chemistry
GeneralExample 2 A food product is made from soybean meal 50 protein 10 moisture and corn flour 10 protein 12 moisture The soybean meal and corn flour are blended together in a 2 1 ratio weight basis Through the manufacturing process there is a loss of water from the system The objective is to make 120 kg h of the final product with a protein content of 40 i Calculate the amounts of soybean meal and corn flour that must be used i Calculate the moisture content of the final product

Physical Chemistry
General2 NaCIO3 2NaCl 302 1 If you have 68 22 g of NaClO3 what is the theoretical yield in liters of oxygen
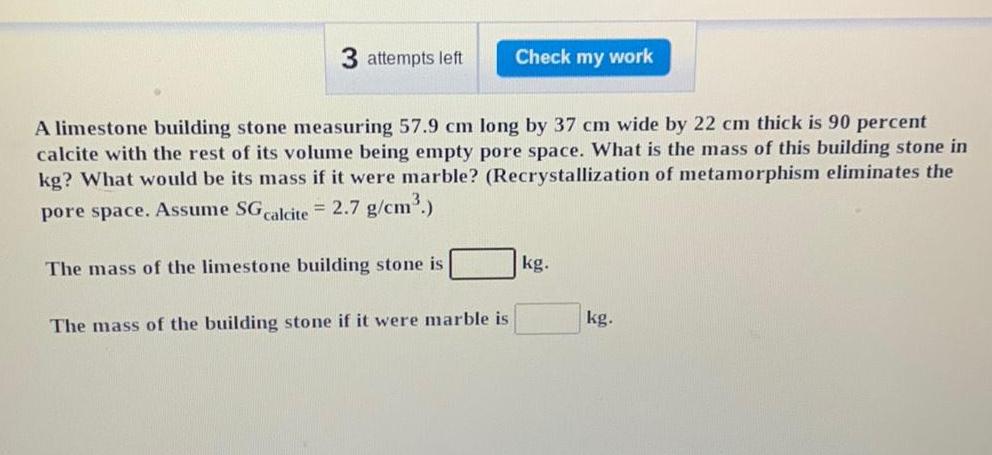
Physical Chemistry
General3 attempts left Check my work A limestone building stone measuring 57 9 cm long by 37 cm wide by 22 cm thick is 90 percent calcite with the rest of its volume being empty pore space What is the mass of this building stone in kg What would be its mass if it were marble Recrystallization of metamorphism eliminates the pore space Assume SG calcite 2 7 g cm The mass of the limestone building stone is The mass of the building stone if it were marble is kg kg

Physical Chemistry
GeneralType of reaction Double displacement reaction Predict the products AgCl Hgl 2 13 Tricarbon octahydride combines with oxygen gas Type of reaction Combustion reaction Predict the products C02 g H 0 14 Solid Barium chloride is mixed with sulfuric acid Type of reaction Double displacement reaction Predict the products Ba S04 HCl 15 Solid Magnesium metal is placed in a solution of nitric acid Single Type of reaction Displacement reaction Predict the products Mg NO3 2 H 16 Sodium phosphate and Copper II chloride react Type of reaction Double displacement reaction Predict the products 17 AlBr3 Cl2 Type of reaction S

Physical Chemistry
General3 attempts left Check my work Express the answer in scientific notation An ore deposit consisting of calcite and galena occurs in a vein measuring 522 m long by 123 m wide by 27 m high The deposit has an average specific gravity of 3 4 How many tonnes of lead can potentially be obtained from the mine Specific gravity can be converted to density by multiplying by the density of water Assume SG galena 7 5 SG calcite 2 7 and g cm tonne m The mass of lead that can potentially be obtained from the mine is x 10 tonnes

Physical Chemistry
General3 2 points Material that itself is not magnetic but attracts to the magnetic field is diamagnetic True False

Physical Chemistry
GeneralRi A M 0 300 0 300 0 150 B M 0 180 0 360 0 180 Rate M min lyr 0 0234 0 0934 C g 2 D g is 0 0234 To the reaction A g 3 B g A Rate K A B B Rate K B C Rate K A B D Rate K A B 63 F Sunny 3 36 PM 4 30 2022

Physical Chemistry
Generalmoles of CO2 how much C6H12O6 can be produced Use the editor to format your answer Question 4 You have 13 2 mols of CO Calculate the mass of CO2 Use the editor to format your answer Question 5 18 6 1 Point 1 Point

Physical Chemistry
GeneralWhat is the net charge on Leu at neutral pH for the dominant ionic species Note The isoelectric point of leucine is 5 98

Physical Chemistry
General5 2 points Kinetic molecular theory assumes gas molecules exert attractive forces and repulsive forces on each other to propel themselves in continuous motion True False

Physical Chemistry
GeneralFor each entry on the left pick the best description on the right acid hydrolysis fat fatty acid unsaturated Clear All a carboxylic acid with a long chain hydrocarbon group containing one or more C C bonds triglycerides that are solid at room temperature converts a triglyceride to glycerol and fatty acids

Physical Chemistry
General2 points A gas at STP refers to temperature of 0 C and pressure of 101 325 kPa True False

Physical Chemistry
General2 points Given the following molecular orbital electron configuration 015 0 15 025 0 25 02px 112py T12pz 4 T 2py T 2pz What is the bond order of this configuration Type your answer

Physical Chemistry
GeneralA linear polysaccharide of glucose monomers is Oglycogen O cellulose O amylopectin O chitin

Physical Chemistry
GeneralA system containing 0 050 mol of a monatomic ideal gas undergoes the process shown t the indicator diagram Notes 100 J 1 bar L R 0 08314 L bar mol K 8 314 J mol K a For each step calculate AS b For each step calculate AH c For each step calculate AG d Calculate AGtot AAtot AStot AHtot AU tot P bar 0 500 a 2 2 50 T Tc T b YO 3 4 25

Physical Chemistry
General2 points Given a fixed amount of gas its pressure is directly proportional to its temperature at constant volume True False

Physical Chemistry
GeneralA radioactive element has a half life of 30 years What is its continuous rate of decay Number round to two decimal places Enter your answer as a positive number

Physical Chemistry
General25 2 points A manometer is device that can be used to measure the atmospheric pressure True False
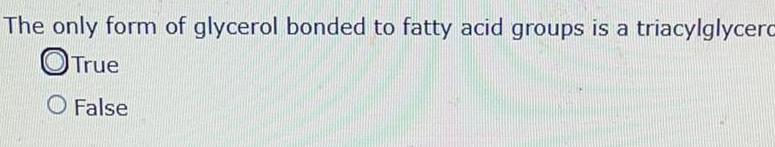
Physical Chemistry
GeneralThe only form of glycerol bonded to fatty acid groups is a triacylglycerc True O False

Physical Chemistry
GeneralK nces Which element is a metal Multiple Choice O O More than one of the elements is a metal Li Si

Physical Chemistry
Generalempis tent Express the answers in scientific notation Granite boulders with a minimum mass of 1 17 tonnes are being used to stabilize a beach What is the minimum volume of a suitably sized stone in cubic meters If the stones are spherical what would be the diameter of the stones in cm Assume SG 2 75 and g cm tonne m granite The minimum volume of a suitably sized stone is The diameter of the stones is x 10 neck my work cm x 10 m

Physical Chemistry
GeneralGlycerol could react with an excess of which of the following to form a fat or an oil Choose all that apply CH N CH3 2 CH3 0 CH3CHCHCOH CH3 CH3 CH 4 CH CHCH 4 CH 2COOH CH3 CH3CHCHCH OH CH3 OCH3 CH 10 COOH

Physical Chemistry
General0 4 points The combustion reaction of ethane gas C H6 g molar mass 30 068 g mol produces CO2 g and H O g according to the chemical equation 2C2H6 g 7O2 g 4 CO2 g 6 H2O g If 37 7 g of C H6 g is burned in excess of oxygen how many liters in L of CO g is released into the atmosphere at 25 C and 1 atm Given R 0 08206 L atm mol K Answer to 1 decimal place Type your answer

Physical Chemistry
GeneralTemperature C 9 1 The following graph represents the heating curve of a hypothetical substance 125 100 25 50 50 KHO 75 25 0 2 4 6 8 Heat added kcal mol What it the melting point 10 12 A 8 401

Physical Chemistry
GeneralWhat is the dominant charge on the side group of Asn at pH 7 Note The pl for Asn is 5 41

Physical Chemistry
GeneralThe hypothetical compound X has molar mass 49 42 g mol and vapor pressure of 626 mmHg at 24 C 50 0 g of coumpound X are introduced in a 15 0 L evacuated flask sealed and left to rest until the liquid reaches equilibrium with its vapor phase What will the mass of the liquid be once equilibrium is reached Insert your answer rounded to 1 decimal digit Answer

Physical Chemistry
GeneralA sample of a metal has a mass of 14 5 g When the sample is placed in a graduated cylinder that contains 32 3 mL of water the volume changes to 36 4 mL What is the density of the metal O the density cannot be determined from this information 4 1 g cm 2 83 g cm

Physical Chemistry
GeneralAnd for Cs Write your answers beginning from and including Xe but without spaces or superscripts So for example Xe 6s26p4

Physical Chemistry
GeneralWhich disaccharide is this ball stick O lactose sucrose O maltose labels None of the Above 14

Physical Chemistry
GeneralWhite a CER Abstract paragraph that answers the question What is the formula for the iron containing compound formed when copper II sulfate reacts with pure iron Be sur to include a clear claim that directly answers the question specific numerical data as well as a brief description of how you obtained that data as evidence and a logical reasoning that clearly explains why the evidence proves your claim Since this lab includes a mathematical process for determining the answer your reasoning should include a step by step explanation of this process Complete sentences and paragraph format are required

Physical Chemistry
General2 points Dutch scientist Johannes van der Waals improved the ideal law by account of real molecules have actual volume and attractions to each other True False

Physical Chemistry
General30 5 points Use Molecular Orbital Theory to predict whether each of the following diatomic ions are stable or unstable a Na 2 b Mg22 c Al 2 d Si 2 e Ar 2 Stable Possible answers HIHAR 88 b No Answers Chosen e d Unstable No Answers Chosen

Physical Chemistry
GeneralWhat is the hybridization of the P atom in each of the following chemical formula a PO4 b b PC15

Physical Chemistry
GeneralCONCLUSIONS Please answer the following questions in complete sentences that incorporate the questions 1 Which of the observations that you made during procedures step 6 could be used as evidence that a chemical reaction has occurred in the experiment