General Questions and Answers

Physical Chemistry
GeneralD 1 42 43 2 points What kind of reaction is the one shown below Fe304 43 44 45 ionic combination decomposition oxidation Previous

Physical Chemistry
GeneralA reaction takes 10 min for 10 complection at 27 C The time taken for 10 completion at 47 C is nearly Eactivation 23 03 kcal mol R 2 cal K mol Antilog 25 24 10 min

Physical Chemistry
General43 2 points When placed in a solution of AgNO3 a piece of copper reacts to form silver metal and copper nitrate Is this a chemical or physical change Chemical Physical Previous

Physical Chemistry
General35 36 37 38 39 40 41 42 2 points The actual yield cannot exceed the theoretical yield O True False Previous

Physical Chemistry
General1 N g 3 H2 g 2 NH3 9 5 If 0 052 mol N are reacted how many mol NH3 are formed Using dimensional analysis show how you calculated your answer 6 If 0 052 mol N are reacted how many mol H are used 7 If 0 863 mol NH3 are produced how many mol N2 must have reacted How many mol H must have reacted

Physical Chemistry
General3 When acetylene C2H2 burns in oxygen high temperatures are produced that are used for welding metals How many grams of CO2 are produced when 54 6 g of C2H2 is burned C2H2 0 CO2 H O

Physical Chemistry
General19 2 points In the reaction below what coefficient should be placed in front of Ca in the balanced equation Ca Sg 10 O O O O 6 00 4 8

Physical Chemistry
General4 2 points The amount of a product that is obtained in a chemical reaction conducted in a laboratory is called the actual yield 0 0 0 0 theoretical yield laboratory yield percent yield Previous

Physical Chemistry
GeneralNumber of hookworms x 24 5 45 88 80 63 Blood lost per day y 12 2 mL 2 48 cm 1 37 inch 1 49 oz 39 8 cm 31 5 mL First examine the data and note the variety of units In order to work with this data it must all be converted to the same unit Convert the data so that all the volumes are reported in the SI unit of milliliters then re enter all the volume data in milliliters in the table below Be sure to report the measurements with the correct number of significant figures Report Table EX 2 Converted Data Set B

Physical Chemistry
General2 In this experiment you will react chromium III with acetylacetone to produce chromium III acetylacetonate The balanced chemical equation is shown below Cr 3 acetylacetone Cr acac a Acetylacetonate acac has a molar mass of 99 11 g mol Use this information and your periodic table to calculate the molar mass of Cr acac molar mass of Cr acac b Determine the limiting reactant when 0 150 g of CrCl3 6H O reacts with 1 5 mL of acetylacetone Make sure to show your work It may be helpful to review CHEM 220 Activity 18 CrCl3 6H O 266 44 g mol Acetylacetone CsH8O 100 12 g mol density 0 975 g mL limiting reactant c Calculate the theoretical yield of Cr acac in grams for the reaction of 0 150 g of CrCl3 6H O and 1 5 mL of acetylacetone Make sure to show your work Review CHEM 220 Activity 18

Physical Chemistry
GeneralA health care provider s prescription reads to administer an intravenous IV dose of 400 000 units of penicillin G benzathine Bicillin The label on the 10 ml ampul sent from the pharmacy reads penicillin G benzathine Bicillin 350 000 units mL The nurse prepares how much medication to administer the correct dose in ml Report answers with appropriate decimal places for professionals to dispense


Physical Chemistry
General4 What is the least soluble substance in 100 grams of water at 10 degrees Celsius 5 What is the molarity of a solution when 2 moles of solute are dissolved in 500 mL of solution 6 How many grams of NaOH s are required to make 2 0 L of a 3 0 M NaOH solution 7 How many mL of 12 0 M HCl aq must be diluted with water to make exactly 500 mL of 3 0 M HCI 8 An 18 M solution of sulfuric acid is diluted from 2 0 L to 6 0 L What is the new molarity



Physical Chemistry
General3 Use the equation below to perform the calculations that follow You must show your work including your set up 3 Ni NO3 2 aq 2 NH4 3PO4 aq 6 NH NO aq Ni3 PO4 2 s a Which type of chemical reaction is illustrated above b Which salt is the precipitate in the reaction above give the chemical formula and the chemical name c Calculate the mass of ammonium nitrate that can be produced by the complete reaction of 0 752 moles of ammonium phosphate 2 0 50


Physical Chemistry
Generaler the questions below about the highlighted atom in this Lewis structure H 0 H C C 0 H I H now many sigma bonds does the highlighted atom ticipate How many pi bonds does the highlighted atom participate at is the orbital hybridization of the highlighted atom 0 0 8 X

Physical Chemistry
General4 Write the molecular equation and then net ionic equation for the neutralization of nitrous acid by sodium hydroxide in aqueous solution

Physical Chemistry
General3 A tire has a pressure of 1 8 atm at 20 degrees Celsius At the end of a long trip the tire pressure increased to 2 0 atm What was the temperature of the air inside the tire in degrees Celsius

Physical Chemistry
Generald sodium chloride and iron II nitrate e aluminum sulfate and sodium hydroxide

Physical Chemistry
GeneralWhen the following reaction takes place which of these better describe the product CH OH A mixture of diastereomeric ethers A mixture of enantiomeric ethers A single chiral compound A mixture of diastereomeric alcohols

Physical Chemistry
General3 Thinking about Earth what factors do you think generate these regular climate cycles

Physical Chemistry
GeneralWhat is the volume in liters occupied by 2 49 g of Freon 12 gas CCl F at 1 86 atm and 35 C Volume L Referenc

Physical Chemistry
GeneralIf 120 ml of a 1 5 M solution of NaOH is diluted to a volume of 225 ml what is the olution s new concentration

Physical Chemistry
General1 The most common health effect of radon is a Respiratory failure b Heart failure c Lung cancer d Mesothelioma

Physical Chemistry
GeneralUse the following table of bond energies to calculate the molar enthalpy of combustion in kJ mol of acetylene C H gas in oxygen based on the following chemical equation C H g 2 502 g 2CO g H O g C Single H Bond H 432 C ONO O Multiple Bonds 411 386 459 N 346 305 167 358 201 C C 602 C C 835 C N 615 C O C O 0 0 O 142 799 1072 494

Physical Chemistry
General11 True or False The cardiac and smooth muscles are controlled by the autonomic nervous system A True B False

Physical Chemistry
GeneralThe equilibrium constant for a given reaction 1 0 x 10 8 What does that indicate about the reaction at equilibrium O The equilibrium lies far to the left and there is a greater concentration of products than reactants The equilibrium lies far to the right and there is a greater concentration of reactants than products O The equilibrium lies far to the left and there is a greater concentration of reactants than products O The equilibrium lies far to the right and there is a greater concentration of products than reactants

Physical Chemistry
General1 Show all of your work and include units 1 Using the actual masses of CuSO 5H O copper II sulfate pentahydrate and Fe iron filings actually used in your experiment how many grams of Cu would be expected to be made if iron II sulfate were formed Which reactant would be limiting Please note that we used copper II sulfate pentahydrate which has a molar mass of 249 72 g CuSO 5H O 1 mol CUSO Note that this does not affect the balancing of the equation nor the mole ratio only the starting point and its molar mass CUSO Fe CUSO Fe Cu FeSO ad Cu 1 FeSO4

Physical Chemistry
General7 If 76 1 grams of NaCl are dissolved in 34 0 mL of Sulfuric acid sulfuric acid has a density of 1 84 gram per mL calculate the following concentration measures 3 points for a and b 4 points for c 10 points total a Weight volume percent b Weight weight percent c Ob g c Molarity Bu are Clin

Physical Chemistry
GeneralA 2 5 M 500 0 mL solution is diluted with water to a new volume of 800 0 mL What is the new molarity at that volume Round to the tenths place

Physical Chemistry
GeneralIf the value of the blank solution is equal to 7 0 g cm3 while the salt concentration is 9 7 g cm3 What will be the value expected on SETA salt analyzer 11 08 PM

Physical Chemistry
General3 What is it responsible for when it comes to the heart A The dependable thump B Circulating Blood C Circulating Oxygen D Circulating Carbon Dioxide

Physical Chemistry
Generalhese equations represent precipitation reactions Rewrite them as complete ionic equations Include phase notations AgNO aq KCl aq KNO aq AgCl s complete ionic equation AgNo Ku AgU KNo Incorrect Ba CIO aq K SO aq BaSO s 2 KCIO aq complete ionic equation Ba So BaSo

Physical Chemistry
General6 What does the smooth muscle line A The heart B The bones C Organs like the intestines and uterus D The brain

Physical Chemistry
Generalthe following groups of atoms in order of increasing s multiple answers as a comma separated list a Rb Na Be b Br Ne Rb c Fe P O

Physical Chemistry
General4 What is the concentration in Molarity of NaCl when 32 0 g are dissolved L in water and diluted to 0 50

Physical Chemistry
GeneralAssign oxidation states to the species in the unbalanced redox reaction Ag aq Cu s Ag s Cu aq Ag aq Cu s Which substance gets oxidized Ag aq Cu s Cu aq Ag s Balance the redox reaction Ag s Cu aq Which substance gets reduced Cu s Ag s Cu aq Ag aq
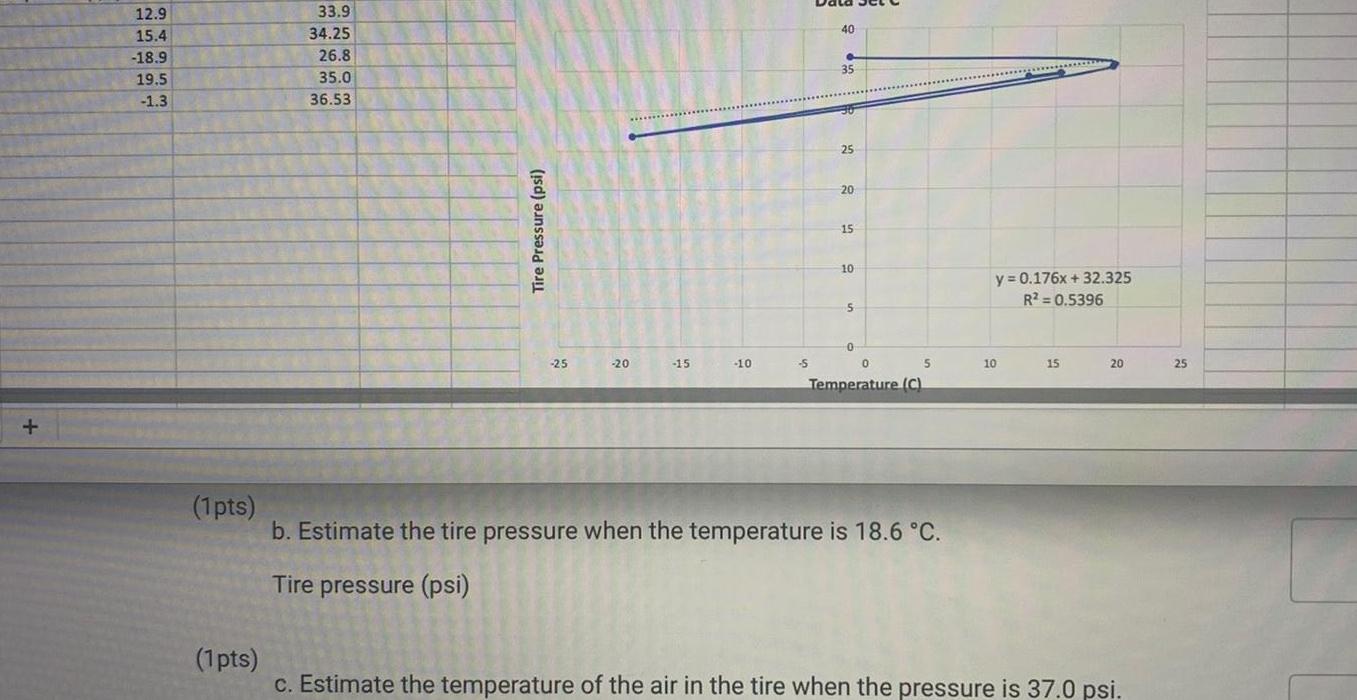
Physical Chemistry
General12 9 15 4 18 9 19 5 1 3 1pts 33 9 34 25 26 8 35 0 36 53 Tire Pressure psi 25 20 15 10 S 40 35 25 20 15 10 5 0 0 5 Temperature C b Estimate the tire pressure when the temperature is 18 6 C Tire pressure psi y 0 176x 32 325 R 0 5396 10 15 20 1 pts c Estimate the temperature of the air in the tire when the pressure is 37 0 psi 25

Physical Chemistry
General5 2 points In the following reaction the reducing agent is 4 Li3P 3 0 6 Li O P4 P4 O O O 02 Lit 02 P3

Physical Chemistry
GeneralO find 1 CH MgBr H O 2 HCO3 H Product CH CH CH C H Number of optically active form of product is X Number of optically inactive form of product is Y X Y

Physical Chemistry
GeneralCircumference cm 25 20 15 10 5 0 0 1 2 3 Data Set A 4 Diameter cm 5 y 3 0004x 0 3284 R2 0 9968 6 7 8 1 pts a Visually estimate the circumference in centimeters of a circle when the diameter is 4 50 cm Circumference cm 1 pts b Calculate the circumference in centimeters for d 4 50 cm using the equation of the best fit line Use the graph to ensure that this value is reasonable Circumference cm B E 1

Physical Chemistry
GeneralWhat is the difference between ribose and deoxyribose Drag the terms on the left to the appropriate blanks on the right to complete the sentences carbon 2 an OH group carbon 3 carbon 1 carbon 4 only OH groups an COOH group only H Ribose has on but deoxyribose has

Physical Chemistry
Generalequation ZnS NO Zn S NO and balance the reaction in acidic solution Which element is oxidized OS ON Ozn Which element is reduced Os ON O zn Which species is the oxidizing agent ZnS O NO Zn Which species is the reducing agent ZnS O NO Zn

Physical Chemistry
General1 5 16 47 48 49 2 points Which of the following is NOT an example of potential energy translational electrical mechanical gravitational Previous


Physical Chemistry
General7 A 1 1000 w v solution is required by a patient On hand in the pharmacy is 20 mL of a 1 250 w v solution How many mL of the 1 1000 w v solution can be made from the stock solution

Physical Chemistry
General3 Which has a higher molarity 1 50 g KCI in 0 250 L or 150 g NaBr in 0 50 L
