General Questions and Answers

Physical Chemistry
GeneralWhich of the following breaks down a
compound into simpler substances?
Synthesis reaction
Addition reaction
Combination reaction
Decomposition reaction

Physical Chemistry
GeneralA supersaturated solution is formed when a solution is holding more solute than it would normally hold at that temperature. How can you make a supersaturated solution of a solute?

Physical Chemistry
GeneralMany claims are made about which foods are the best for us to eat. What are some characteristics you can look for to distinguish valid scientific evidence from a claim without such evidence?

Physical Chemistry
GeneralRank the following bonds from the strongest to the weakest. Make 1 the strongest and 6 the
weakest.
Van der Waal Forces
Ionic Bonds
Single Covalent Bonds
Double Covalent Bonds
Hydrogen Bonds
Triple Covalent Bonds

Physical Chemistry
GeneralRetired people like to invest in shares and stocks with the object of investment. This is because of
steady dividend Income
risk aversion
low capital gain

Physical Chemistry
GeneralAre the following Ionic, Nonpolar Covalent, Polar Covalent, or Metallic?
a. Bonds by evenly sharing electrons
Bonds by transferring electrons
b.
c. Bonds by unevenly sharing electrons
d.
Dissolves in water, conducts when dissolves, high
melting point.
Doesn't dissolve in water, conducts in the solid
state, malleable, ductile, lustrous.
f.
Doesn't dissolve in water, doesn't conduct, low
melting points.
g. Dissolves in water, doesn't conduct current.
Metal bonded to a nonmetal
h.
i.
j.
Nonmetal bonded to a nonmetal
Metal atoms bonded to metal atoms.


Physical Chemistry
GeneralIf a person has 97mol of K2O,
what mass, in grams, of K₂O do
they have? Round to two decimal
places.

Physical Chemistry
GeneralA person combines 9g of A with some reactant B
to form 28g of C and 8g of D according to the
reaction below. Assuming the reactants were
completely used up, what mass of reactant B was
reacted?
A+B-->C+D

Physical Chemistry
GeneralIf we have added the maximum amount of solute to be dissolved in a solvent at a particular temperature, what type of solution have we made?

Physical Chemistry
GeneralA person mixes 1mol of magnesium metal with
1.5mol of hydrochloric acid (HCI). The reaction
below occurs. What is the limiting reactant for the
reaction?
Mg(s) + 2HCl(aq) --> MgCl2(aq) + H2(g)
Magnesium
There is no limiting reactant here.
Hydrochloric Acid (HCI)
Magnesium Chloride
Hydrogen Gas


Physical Chemistry
GeneralKnow how to write the names and formulas for ionic and covalent compounds. While there are NO
specific naming questions - you will see names when completing reactions.
a. Write the names for the following compounds:
i. S406
iii. KOH
ii. Fe3(PO4)2
iv. CO
b. Write the formulas for the following compounds:
i. nitrogen
ii. potassium fluoride
iii. Lithium sulfite
Which 3 elements are "special"? What makes them special and what are their charges?
iv. cesium hypochlorite
v. Tricarbon octahydride
vi. Iron (II) oxide

Physical Chemistry
GeneralIn one of the reactions in the citric acid cycle, which provides energy,
succinic acid is converted to fumaric acid.
C4H6O4 → C4H4O4 + 2H
Succinic acid Fumaric acid
The reaction is accompanied by a coenzyme, flavin adenine dinucleotide
(FAD).
FAD+2H --> FADH₂
a. Is succinic acid oxidized or reduced?
b. Is FAD oxidized or reduced?
c. Why would the two reactions occur together?

Physical Chemistry
GeneralThe chemical property of an acid is its __ boiling point melting point density reactivity

Physical Chemistry
GeneralClassify each of the following as exothermic or endothermic: a. The energy level of the products is lower than that of the reactants. b. In the body, the synthesis of proteins requires energy. c. A reaction absorbs 125 kJ.

Physical Chemistry
GeneralCalculate the molar mass for each of the following: a. 02 b. KH₂PO4 c. Fe(ClO4)3

Physical Chemistry
GeneralWhich of the following mole ratio corresponds to the mole ratio of calcium to calcium chloride for the reaction 2Ca(s)+ 2Cl₂ (g) → 2CaCl₂(s)? 2:1 1:2 1:1 4:1

Physical Chemistry
GeneralFor Nitrogen Trifluoride: -Draw its structure (including labeling any partial charges if present) -What Shape is the Molecule? - Are the individual bonds Polar or Nonpolar? -Is the molecule as a whole Polar or Nonpolar?

Physical Chemistry
GeneralWhich two things affect how a lens refracts light? A. The location of the image B. The curvature of the two sides of the lens c. The material the lens is made from D. The material the object is made from

Physical Chemistry
General.How many iodide ions do you think might be bonded to each lead ion in lead iodide? (Give the reason for your answer, if you can.)

Physical Chemistry
GeneralIn the equation aCH4 + bO2---> CCO₂ +dH₂O, which of the following corresponds to a, b, c, and d, respectively, to make the equation balanced?. 1, 2, 1, 2 2, 1, 1, 2 2, 2, 1, 1 1, 1, 2, 2

Physical Chemistry
GeneralWhat happens when milk is converted into curd? The quantity of curd and whey produced is greater in comparison to the milk. The quantity of curd and whey produced is less in comparison to the milk. The quantity of curd and whey produced is same as the quantity of the milk. None of the choices

Physical Chemistry
GeneralAn engineer turns the temperature of a gas down, holding the volume constant at 1,250 L. Initially, the temperature of the gas was 601.°C and had a pressure of 274. kPa. Determine the pressure of the gas when the temperature decreased to 157°C.

Physical Chemistry
GeneralWhich of the following coefficients will balance the equation NaN3 (s) Na(s) + N₂ (g), respectively? 1, 1,3 1,2,3 1, 3, 2 2, 2, 3

Physical Chemistry
General1.) PC15(s)+H2O(1) POCI3(1)+2HCl(aq) When 53.22 g of phosphorus pentachloride reacts with excess water, what mass of hydrogen chloride will be produced? 2.) Determine the molarity of a solution with a volume of 758. mL and 0.620 mol of solute dissolved.

Physical Chemistry
GeneralIdentify the correct statement from the following. Stirring increases the rate of dissolution. Pressure is only applicable for solid solutes. Any solute can be dissolved in any solvent. solute dissolves solvent to form a solution.

Physical Chemistry
GeneralUse LeChatelier's Principle to answer the following set of questions:
2NO(g) + O2(g) << 2NO2(g) + heat
a. For the reaction shown above, what would happen to the concentration of reactants if pressure were increased on the system?
b. If the NO₂ is increased, how will the equilibrium be affected? What will happen to the concentration of the oxygen gas?

Physical Chemistry
GeneralWrite the names for the following compounds:
i. S406
ii. Fe3(PO4)2
iii. KOH
iv. CO

Physical Chemistry
GeneralPick the odd one out.
HCI (aq) + NaOH (aq) → NaCl (aq) + H₂O (1)
HCI (aq) + 2 NaOH (aq) → NaCl (aq) + 2 H2O (I)
HCI (aq) + 2 NaOH (aq) → 2 NaCl (aq) + H2O (I)
2 HCI (aq) + NaOH (aq) → NaCl (aq) + 2 H2O (I)

Physical Chemistry
GeneralWhich of the following equations best describes the law of conservation of mass?
KCl(aq) + AgNO3(aq) AgCl(s) + KNO3(aq)
H₂ (g) + O₂ (g)→ H₂O₂ (g)
H₂O₂ (g)→ H₂O(g) + O₂(g)
KCl(aq) + Ag₂NO3 (aq) → AgCl(s) + KNO3(aq)

Physical Chemistry
GeneralIdentify the molecule containing the least oxygen atoms.
12.044 x 1023 molecules of CO₂.
12 g of 03.
0.1 mol of V₂05.
10 mL of H₂O.

Physical Chemistry
GeneralFor a Nitrogen Molecule
-Draw its structure (including labeling any partial charges if present)
-What Shape is the Molecule?
-Are the individual bonds Polar or Nonpolar?
-Is the molecule as a whole Polar or Nonpolar?
-

Physical Chemistry
GeneralExplain why Gasoline (C8H18) evaporates so easily from an intermolecular force point of view.

Physical Chemistry
GeneralFor Boron Trichloride:
-Draw its structure (including labeling any partial charges if present)
- What Shape is the Molecule?
- Are the individual bonds Polar or Nonpolar?
-Is the molecule as a whole Polar or Nonpolar?

Physical Chemistry
GeneralConsider the following observed reactivity information. Rank the elements based on their strength as reducing agents (i.e. strongest to weakest reducing agent). Explain your thinking. (4 marks)
Sn + 2AgBr➜ SnBr2 + 2Ag
3Sn + 2AuBr3 → 3SnBr2 + 2Au
3Ag + AuBr3 → 3AgBr + Au

Physical Chemistry
General26. How much energy is absorbed when 129 g 2-propanol (structure shown) is vaporized at its
normal boiling point of 82.3°C? The AHvap of 2-propanol is 39.9 kJ/mol at 82.3 °C and its
structure is shown here.
a. 85.6 kJ
b. 5,150 kJ
c. 85.649 kJ/mol
d. 5.15 x 103 kJ
HHH
|||
H-C-C-C-H
|
|
:0: H
T
H
|
H

Physical Chemistry
General4K(s) + O2(g) →→→ 2 K₂0(s)
If a staff member at a laboratory has 12.0 grams of potassium metal, what is the
theoretical yield of potassium oxide that the scientist could potentially produce
from the reaction with oxygen gas?
A) 9.96 grams of K₂0
B) 14.5 grams of K₂0
C) 28.9 grams of K₂O
D) 57.8 grams of K₂0

Physical Chemistry
GeneralThe reaction of charcoal (carbon) and oxygen is
sped up by grinding the charcoal into a fine
powder. This is an example of:
O Increasing temperature to increase reaction rate
O Increasing concentration to increase reaction rate
increasing surface area to increase reaction rate
O All of the above

Physical Chemistry
GeneralThe half-life of krypton-91 (Kr) is 10 s. At time t= 0 a heavy canister contains 4 g of this radioactive gas.
(a) Find a function m(t) = m 2-t/h that models the amount of 91Kr remaining in the canister after t seconds.
m(t) =
1
(2) 10
(b) Find a function m(t) = moet that models the amount of 91Kr remaining in the canister after t seconds. (Round your r value to five decimal places.)
m(t) =
(c) How much 91Kr remains after 1 min? (Round your answer to three decimal places.)
►
(d) After how long will the amount of 91Kr remaining be reduced to 1 µg (1 microgram, or 10
-6
g)? (Round your answer to the nearest whole number.)
sec

Physical Chemistry
General7. If 76.1 grams of NaCl are dissolved in 34.0 mL of Sulfuric acid (sulfuric acid has
Sa density of 1.84 gram per mL), calculate the following concentration measures.
(3 points for a and b, 4 points for c 10 points total)
a. Weight/volume percent
b. Weight/weight percent
c. Molarity

Physical Chemistry
General+6-6-6
ons: Complete the following reactions that are happening in water. Make sure
to tell the states of matter for all compounds involved. If the reaction is not possible
state that.
3.
4.
5.
5.
3.
HCI + Na
Pb(NO3)2 + KOH
_AI(NO3)3 + Zn
NaOH + K₂S
LiC2H302 + NaCl
-

Physical Chemistry
GeneralRadish extract indicator turns to a bright red color in low pH solutions and mustard-yellow in solutions with a high pH value.
If a teacher places this radish extract indicator in a NaOH water solution, which of the following accurately explains what will happen?
The indicator will not turn colors because there is an equal ne
The indicator will turn bright red becau
Gure H" than OH ions, and the solution is acidic.
The indicator will turn bright because there are more H* ions than Na* ions dissolved in water, and the solution is acidic
The indicator will turn mustard-yellow because there are more OH than H* ions, and the solution is basic.
A
B
C
D
Na and OHT ions dissolved in water, and the solution is neutral.

Physical Chemistry
GeneralA student places 200.0 g of water at 49.70°C in a calorimeter. The student then adds
200.0 g of water at 3.20°C to the calorimeter. The temperature after mixing was 28.50°C.
What is the value of the calorimeter constant?

Physical Chemistry
GeneralA 1.628g sample of a hydrate
of Magnesium iodide (Mgl₂ xH2O) was
heated strongly for five minutes. The
mass of the anhydrous left had a mass
of 0.712g. It was then heated for a
further 3 minutes and the mass
remained at 0.712g.
a. Find the mass of water driven off (1
mark)
b. Find the moles of anhydrous magnesium
iodide and moles of water (3 marks)
c. Find the name and formula of the
hydrate (2 marks)
d. Why was the compound heated twice? (1
mark)

Physical Chemistry
GeneralA scientist repeats the Millikan oil drop experiment in a different galaxy and the charge on the drops is measured in a unit called
the electrino (el). The scientist obtains data for four drops.
Drop
A
B
Calculated charge (el)
5.52 x 10-14 el
2.30 x 10-14 el
3.22 × 10-14 el
3.68 x 10-14 el
Given the results of the scientist's experiment, determine the charge on an electron in electrinos.
C
D
electron charge:
Table
Given that the charge on an electron is 1.60 x 10-19 C, determine the conversion factor between electrinos and coulombs.
IC=
el
el
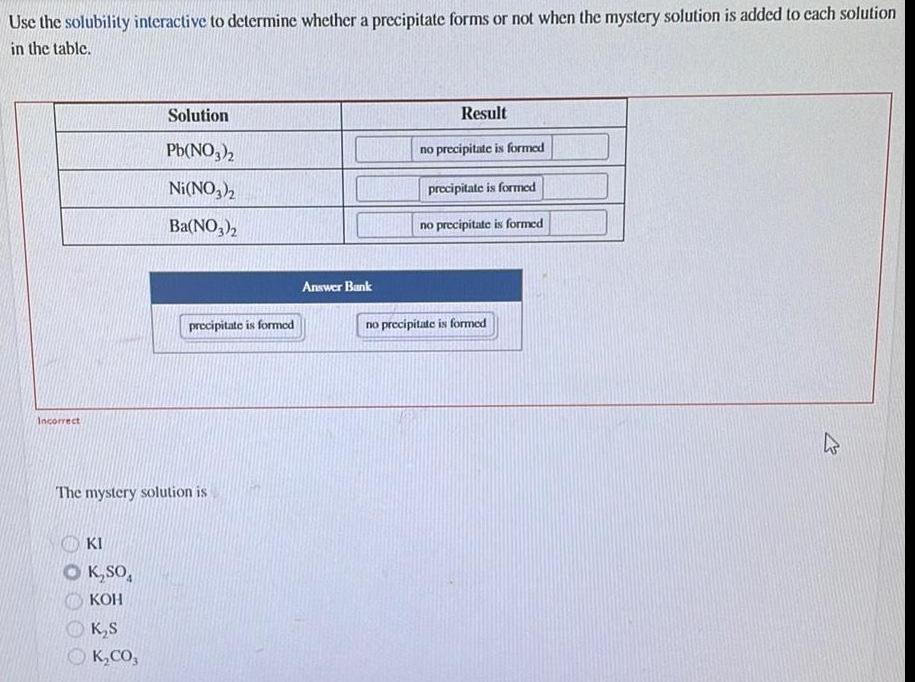
Physical Chemistry
GeneralUse the solubility interactive to determine whether a precipitate forms or not when the mystery solution is added to each solution
in the table.
Incorrect
KI
O K₂SO4
KOH
Solution
Pb(NO3)2
Ni(NO3)2
Ba(NO3)2
The mystery solution is
K₂S
K₂CO,
precipitate is formed
Answer Bank
Result
no precipitate is formed
precipitate is formed
no precipitate is formed
no precipitate is formed

Physical Chemistry
GeneralA scientist is investigating the solubility of two polyatomic ions, oxalate (C₂02) and arsenate (ASO), which are not listed in
the table of solubility guidelines.
The scientist starts with four solutions made of water-soluble salts. Solution A contains sodium arsenate. Solution B contains
ammonium oxalate. Soution C contains silver chlorate. Solution D contains aluminum bromide.
Solute
Color of Solution
Na, AsO4
colorless
(NH,),C,O.
colorless
colorless
AgCIO,
AlBr
yellow
The results of mixing each solution in pairs are shown in the table.
Experiment Solutions Mixed
1
A + B
2
A+C
3
A+D
B+C
B+D
C+D
Solution
A
B
C
D
4
5
6
Identify the formula for each precipitate that forms.
precipitate from A+C:
precipitate from B+C:
2
Incorrect
precipitate from C+D:
4
Incorrect
6
Result
no precipitate, colorless solution
brown precipitate
white precipitate
white precipitate
white precipitate
yellow precipitate
Incorrect
precipitate from A+D:
precipitate from B+D:
3
Incorrect
15
Incorrect

Physical Chemistry
GeneralType the correct answer in each box.
Scientists often have to deal with numbers that are either very large or very small. For example, the radius of the Sun Is approximately 696,000
kilometers, while bacterial cells are as small as 1.9 x 104 millimeters. Express each number in an alternate form.
In scientific notation, the radius of the Sun is
In expanded notation, each bacterial cell is
6.96 x 10.
5
kilometers.
millimeters wide.

Physical Chemistry
GeneralAssume there is an equal mass of each gas at a given temperature and pressure. Arrange the gases based on the amount of
volume they occupy, from the largest to the smallest volume.
H₂
SO₂
0₂
X
Largest volume
Ne
Smallest volume
Answer Bank