General Questions and Answers

Physical Chemistry
General1 L
½L
CoCl₂
Concentration
(mol/L)
?
How many moles is 130 g of CoCl₂?
moles
What is the molarity of a solution with 130 g of CoCl₂ in ½ Liter?
How many grams of CoCl₂ in ½ Liter would be used to make a 1.0 molar solution?
grams
(provide answers in 2 significant figures; numbers only)
Molar

Physical Chemistry
GeneralEthanol (C3H6O) is a liquid at room temperature :
1 mole = 6.02 x 1023 atoms
Density of C3H60 = 0.815 %/mL
What is the molar mass of C3H6O = 58
8/mol
How many moles of ethanol are present in 10.4 mL of C3H6O? 0.146
How many carbon atoms are in 10.4 mL of C3H6O? 2640000000000000000000 atoms of C
Show your work:

Physical Chemistry
GeneralA sample of a hydrocarbon is found to contain 7.99g carbon and 2.01g hydrogen. What is the empirical
formula for this compound?
Hint: CH4 enter as CH_4

Physical Chemistry
GeneralConsider the following three solutions of NaOH
P-20 ppm NaOH in 5 kg of water
Q-1000 ppm NaOH in 5000 kg of water
R 0.001 kg NaOH in 5 kg of aqueous solution
Which of the following is (are) correct order(s) of molality of NaOH?
OP<Q
O Q>R
P>Q
OR>P

Physical Chemistry
GeneralHow many liters of a 0.570 M sucrose (C12 H22O11) solution contain 1.3 kg of sucrose?
Express your answer using two significant figures.
VAX
E9ZZ220
?
L

Physical Chemistry
GeneralUse the van der Waals equation of state to calculate the pressure P of 4.00 mol of CO₂ at 453 K in a 5.00 L vessel. Use this list
of van der Waals constants.
P =
Use the ideal gas equation to calculate the pressure P under the same conditions.
P =
atm
atm

Physical Chemistry
GeneralWhat is the total number of moles of solute in 0.305 liters of a 3.91 M solution of NaCl?
mol

Physical Chemistry
GeneralSuppose
a student is performing their
first trial of Reaction 1 (10 mL of 4.0 M
acetone, 10 mL of 1.0 M HCI, 10 mL of
0.005 M iodine and 20 mL of water). If
a student accidentally adds 11 mL of
0.005 M iodine instead of 10 mL of
0.005 M iodine, how will their rate be
effected?
The student's calculated rate will be
faster than the true value.
The student's calculated rate will be
slower than the true value.
The student's rate will not be effected.

Physical Chemistry
GeneralUpon heating 113 g MgSO4.7H₂O:
(a) how many grams of water can be obtained?
i
eTextbook and Media
(b) how many grams of anhydrous compound can be obtained?
Hint
the correct molar masses for water and MgSO4?
GO Tutorial
!
! 8 H₂0
g MgSO4

Physical Chemistry
GeneralA beam of alpha particles is fired horizontally with a speed of 4.5 x 105 m/s into a region where there is a vertical magnetic field
of magnitude 0.337 T. An alpha particle is the nucleus of a helium atom and as such, consists of two protons, two neutrons, and
has a mass of 6.64 x 10-27 kg.
(a) Determine the length of time it will take an alpha particle to move halfway around a complete circle.
S
(b) Does the time found in part (a) increase, decrease, or stay the same, if the speed of the alpha particle is tripled?
increase
decrease
Ostay the same
(c) Repeat part (a) for alpha particles with a speed of 1.4 x 106 m/s.
S

Physical Chemistry
GeneralWe'll begin by calculating the energy absorbed by the water. The water increased in temperature from 21.4 °C to 39.2
°C. What quantity of heat did it absorb?
Recall from the video that 200 mL of water were used and that the specific heat capacity of water is 4.18 J/g °C.
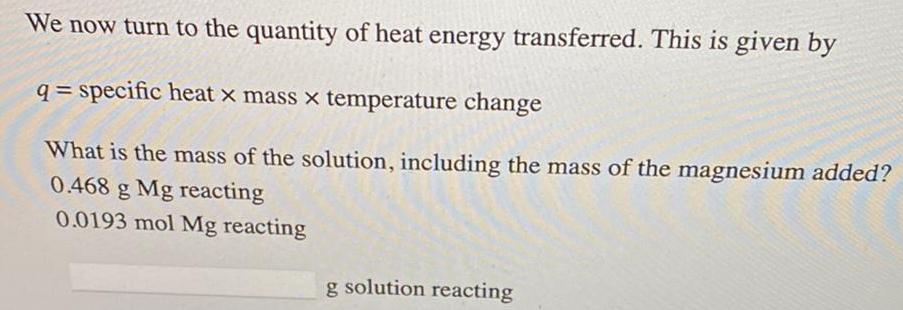
Physical Chemistry
GeneralWe now turn to the quantity of heat energy transferred. This is given by
q= specific heat x mass x temperature change
What is the mass of the solution, including the mass of the magnesium added?
0.468 g Mg reacting
0.0193 mol Mg reacting
g solution reacting

Physical Chemistry
GeneralConsider the combustion reaction, 2CgHgO3(l) + 1702(g) → 16CO2(g) + 8H₂O(l). If stoichiometric amount of C8H8O3 is burnt with 4.25 mol of oxygen,
select the correct statement(s) about the reaction.
If 20% by mole of C8HgO3 is consumed then 0.8 mol of gas is formed
If 20% by mole of CgH8O3 is consumed then 0.4 mol of gas is formed
After 100% completion of reaction 8 moles of gas is formed
After 100% completion of reaction combined moles of product formed are 6

Physical Chemistry
GeneralThis means that the steel bar lost 14900 J of thermal energy. What is the change in temperature of the steel bar? Recall
that the steel decreases in temperature, and use the value of 0.49 J/g °C as the specific heat capacity of steel. The video
shows the mass of the steel to be 40.7 g.
AT=
°C

Physical Chemistry
GeneralChoose the sentence that is punctuated correctly.
However he spared them; in fact he allowed the throne to pass to the young boy.
However, he spared them; in fact he allowed the throne to pass to the young boy.
However, he spared them; in fact, he allowed the throne to pass to the young boy.

Physical Chemistry
GeneralA gas mixture consists of N2, O2, and Ne, where the mole fraction of N₂ is 0.55 and
the mole fraction of Ne is 0.25. If the mixture is at STP in a 5.0 L container, how
many molecules of O₂ are present?
(Enter answer in the space provided below and submit all supporting calculations in your
work upload in order to receive full credit)
Note: Scientific notation should be entered as shown: 2.00 x 108 can be entered as
2.00x10^8 (without any spaces)

Physical Chemistry
GeneralSmall quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KC103 (s).
The equation for the reaction is
2 KClO3
->> → 2 KCl +30₂
Calculate how many grams of O₂(g) can be produced from heating 25.5 g KC1O3(s).
mass:
g 0₂

Physical Chemistry
GeneralChoose the sentence that uses apostrophes correctly.
Heather said the car wasn't hers. Well, it wasn't ours, either.
Heather said the car wasn't hers. Well, it wasn't our's, either.
Heather said the car wasn't her. Well, it wasn't ours', either.

Physical Chemistry
GeneralTo which of the following rules does this example apply?
There were about 500 guests at Richard and Roselle's wedding party.
If there are two or more possessors of a certain object, then the apostrophe is put at
the end of the last noun.
O
If the possessive case is a singular noun or an indefinite noun, then the apostrophe is
put at the end of the noun along with an "s."
O
If the possessive case is a plural noun that end in s, then the apostrophe is put at the
end of the plural noun after the final "s."

Physical Chemistry
GeneralThe standard heat of formation for TiO₂(s) is -940 kJ/mol at 298 K. Write the formation
equation for TiO2 (s) that goes with this value of AH°.
Be sure to specify states. Write fractions with a slash, such as 1/2 for one half. If a box is not
needed leave it blank.

Physical Chemistry
GeneralOcean water contains 3.1% NaCl by mass.
Part A
How much salt can be obtained from 314 g of seawater?
Express your answer in grams to two significant figures.
the Review link

Physical Chemistry
Generaldentify the correctly punctuated sentence below.
This doll is hers'; your's is in the kitchen.
This doll is hers; yours is in the kitchen.
This doll is hers; yours' is in the kitchen.

Physical Chemistry
GeneralChoose the option that shows the correct use of punctuation.
"Good Evening!" said the pilot. "Fasten your seat belts, as we are ready to take off."
O"Good Evening"! said the pilot, "Fasten your seat belts, as we are ready to take off."
O"Good Evening! said the pilot. Fasten your seat belts, as we are ready to take off."

Physical Chemistry
GeneralTo what volume should you dilute 15 mL of a 12 M stock HCl solution to obtain a 0.600 M HCl solution?
Express your answer using two significant figures.

Physical Chemistry
GeneralThe magi, as you know, were wise men - wonderfully wise men - who brought gifts to
the Babe in the manger. They invented the art of giving Christmas presents. Being
wise, their gifts were no doubt wise ones, possibly bearing the privilege of exchange in
case of duplication. And here I have lamely related to you the uneventful chronicle of
two foolish children in a flat who most unwisely sacrificed for each other the greatest
treasures of their house. But in a last word to the wise of these days let it be said that
of all who give gifts these two were the wisest. Of all who give and receive gifts, such
as they are wisest. Everywhere they are wisest. They are the magi. (The Gift of the
Magi by O. Henry)
Summary
O Question
Strong statement

Physical Chemistry
GeneralSome soluble compounds are listed in the table below.
Classify each compound using the checkboxes.
compound
NH₂
NaOH
Ba(OH)₂
Nal
type of compound (check all that apply)
strong weak strong
acid acid base
ionic molecular
X
Ś
weak
base
?

Physical Chemistry
GeneralRead the following and identify which style of writing is it?
"To see Northern lights you have to go out at night, and then reach a place which is at
a higher level."
expository
Ochronological
Sequential

Physical Chemistry
GeneralA 225 mL sample of ocean water contains 7.1 g
of NaCl.
What is the molarity of the solution with respect to NaCl?
Express your answer using two significant figures.

Physical Chemistry
Generalhe samarium-147 nuclide radioactively decays by alpha emission. Write a balanced nuclear chemical equation that describes this process.
0
X 5
Y
?

Physical Chemistry
GeneralA 175 mL sample of a 1.3 M sucrose solution
is diluted to 550 mL.
What is the molarity of the diluted solution?
Express your answer using two significant figures.
VAZO
GIG
M

Physical Chemistry
General(C2H5OHC2H5OH)
Ethanol
melts at -114 °C. The enthalpy of fusion is 5.02
kJ/mol. The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-
K, respectively. How much heat (kJ) is needed to convert 25.0 g of solid ethanol
at -125 °C to liquid ethanol at -40 °C?

Physical Chemistry
GeneralThe salt calcium chloride is soluble in water. When 0.960 g of CaCl₂ is dissolved in 116.00 g of water, the
temperature of the solution increases from 25.00 to 26.51 °C. Based on this observation, calculate the enthalpy of
dissolution of CaCl₂ (in kJ/mol).
Assume that the specific heat of the solution is 4.184 J/g °C and that the heat absorbed by the calorimeter is negligible.
AH dissolution
kJ/mol

Physical Chemistry
GeneralRead the following lines from the essay, "On Reading and Books" by Arthur
Schopenhauer, and answer the question that follows:
"There are at all times two literatures which, although scarcely known to each other,
progress side by side-the one real, the other merely apparent. The former grows
into literature that lasts. Pursued by people who live for science or poetry, it goes its
way earnestly and quietly, but extremely slowly, and it produces in Europe scarcely a
dozen works in a century, which, however, are permanent. The other literature is
pursued by people who live on science or poetry; it goes at a gallop amid a great noise
and shouting of those taking part, and brings yearly many thousand works into the
market. But after a few years one asks, Where are they? Where is their fame, which
was so great formerly? This class of literature may be distinguished as fleeting, the
other as permanent."
What is the purpose of the writer to use this comparison?
To talk about the literates of science and art
O To tell about the types of literature
To make readers understand what good books are

Physical Chemistry
GeneralIf 6.50 L of water vapor at 50.2 °C and 0.121 atm reacts with excess iron, how many grams of iron(III) oxide will be produced?
2 Fe(s) + 3 H₂O(g) - ► Fe₂O3(s) + 3 H₂(g)
mass:

Physical Chemistry
GeneralThe number of pairs in which magnitude of electron affinity of the second element is more than that of the first element is
(O, F) (Si, P) (F, CI) (CI, Br) (O, S) (S, Se) (N, P)
Answer:
00
01
02
O 3
00
01
02
Select your answer from radio buttons.
4
05
6
06
07
07
8
08
9

Physical Chemistry
GeneralMISSED THIS? Watch IWE 13.2; Read Section
13.5. You can click on the Review link to access the
section in your e Text.
Determine the amount of sucrose in each of the
following solutions.
▾ Part A
44 g of a solution containing 4.0 % sucrose by mass
Express your answer using two significant figures.
VAXO
Submit
▾ Part B
Request Answer
599
?
bo
g
115 mg of a solution containing 10.5 % sucrose by mass
Express your answer using three significant figures.
![Consider the following chemical reaction:
Pb(NO3)2 + Na2SO4 → PbSO4 + 2NaNO3
[Molar mass: Pb(NO3)2 = 331, Na₂SO4 = 142]
If a series of experiments are run maintaining sum of the weights of two reactant constant but varying the weight composition of reactants. Which of
the following statement is (are) not true?
E
Maximum weight of the precipitate (PbSO4) will be formed if equal weight of reactants are taken
Maximum weight of the precipitate (PbSO4) will be formed if equal moles of reactant are taken
In the experiment, as the weight of Na₂SO4 increases weight of precipitate (PbSO4) increases indefinitely
In the experiment as the weight of Pb(NO3)2 increases weight of precipitate (PbSO4) increases indefinitely](https://media.kunduz.com/media/sug-question/raw/59683493-1659708625.5588317.jpeg?w=256)
Physical Chemistry
GeneralConsider the following chemical reaction:
Pb(NO3)2 + Na2SO4 → PbSO4 + 2NaNO3
[Molar mass: Pb(NO3)2 = 331, Na₂SO4 = 142]
If a series of experiments are run maintaining sum of the weights of two reactant constant but varying the weight composition of reactants. Which of
the following statement is (are) not true?
E
Maximum weight of the precipitate (PbSO4) will be formed if equal weight of reactants are taken
Maximum weight of the precipitate (PbSO4) will be formed if equal moles of reactant are taken
In the experiment, as the weight of Na₂SO4 increases weight of precipitate (PbSO4) increases indefinitely
In the experiment as the weight of Pb(NO3)2 increases weight of precipitate (PbSO4) increases indefinitely

Physical Chemistry
GeneralChoose the words with properly placed commas to form a correctly punctuated
sentence.
Guided by moonlight Rick could see where the path diverted from the main
road.
see, where
light, from
O moonlight, Rick

Physical Chemistry
GeneralA gas has distribution of molecular speeds at different temperatures as
shown in below figure. Select the correct option about the molecular speeds
of the gas at different temperatures
Fraction of molecules
0.020-
0.015
0.010+
0.005-
100
100 K
200
(D) Ratio of Cmp:
300 K
+ +
300 400
500 K
500
600
speed (m-s¹)
Average speed-c
Most probable speed - Cop
Root mean square speed - Cs
(A) Ratio of c: Cmp is highest at temperature 500 K
(B) Ratio of C: Crms is lowest at temeparture 100 K
(C) Ratio of Cmp Crms at 300 K is same as at 500 K
:
C: Crms is 1: 1: 1 at 100 K

Physical Chemistry
General40. Write out 6.02 x 1023 as a regular number. Use commas within this number to separate the digits
appropriately. (4 pts.)
41. Write a brief paragraph to explain how this diagram relates to the principles of electricity, using the
terms, voltage, current, and resistance appropriately (5 pts.).
Volt
Amp

Physical Chemistry
GeneralCalcium carbonate is a common ingredient in antacids that reduces the discomfort associated with acidic stomach or heartburn.
Stomach acid is hydrocholoric acid, HCl.
What volume in milliliters (mL) of an HCl solution with a pH of 1.52 can be neutralized by 17.0 mg of CaCO3?
volume:
If the stomach contains 14.7 mL of pH 1.52 solution, will all of the acid be neutralized?
yes
no
What percentage of the acid is neutralized? If all of the acid is neutralized enter 100%.
percentage neutralized:
mL
%

Physical Chemistry
GeneralThe table below lists information about the radioactive decay of three nuclides. Fill in the missing information.
nuclide
0
10
4 Be
147
62 Sm
decay mode
alpha emission
(choose one)
alpha emission
daughter nuclide
146
62
Sm
10
B
X
3 ?

Physical Chemistry
GeneralMISSED THIS? Watch IWE 13.4; Read Section
13.6. You can click on the Review link to access the
section in your e Text.
What volume of each solution contains 0.15 mol of
KCI?
Submit
Part C
Request Answer
0.835 M KC1
Express your answer using two significant figures.
VAX
SANO ?
L
L
Review I

Physical Chemistry
GeneralIdentify the strategy used in this conclusion.
As the population continues to age, the demand for medical care will only increase.
Without the support of the federal government to supply health insurance for its
citizens, many people will suffer and die needlessly. Can we let this happen to the
people in this country?
Strong statement
Question
Personal Comment

Physical Chemistry
General0.112 mol of sucrose in 680 mL of solution
Express your answer using three significant figures.
VAE
Submit Request Answer
Part B
?
0.235 mol of KNO3 in 0.885 L of solution
Express your answer using three significant figures.
VAE
?
M

Physical Chemistry
GeneralConsider the reaction of aqueous solutions of FeF3 and AgNO3.
FeF3 (aq) + 3 AgNO3(aq) → Fe(NO3)3 (aq) + 3 AgF (s)
In the lab, you mix 284 mL of 4.5 M AgNO3 and 255 mL of 3.2 M FeF3.
How many moles of AgNO3 do you have?
How many moles of AgF form when you use up all the AgNO3?
How many moles of FeF3 do you have?
mol
mol
How many moles of AgF form when you use up all the FeF3?
How many grams of AgF are expected to form based upon the limiting reactant?
If your actual yield is 85 g AgF, then what is your percent yield?
mol
mol
%

Physical Chemistry
GeneralRe-sketch the diazene molecule and label all bond dipoles (with the arrow pointing from the
most electronegative atom to the least electronegative atom). Show using vector sums that this
geometric isomer of diazene is non-polar.

Physical Chemistry
GeneralDuring a titration, 135.0 mL of H₂CO3 was required to neutralize
115.0 mL of 0.551 M KOH. What is the concentration of the
carbonic acid?
Balance the chemical reaction:
H₂CO3 + KOH-
Answer: -------M H₂CO3
XE DU DR DU AN DAS AO AN

Physical Chemistry
GeneralStudy this chemical reaction:
Al(s)+FeBr3(aq) AlBr3(aq) + Fe(s)
Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
oxidation:
reduction:
FD1
X
00
S
09
?

Physical Chemistry
GeneralNitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, and hydrogen prepared by reforming natural gas, in a two-step process. In
the first step, nitrogen and hydrogen react to form ammonia:
N₂(g) + 3H₂(g) → 2NH₂ (g)
1
In the second step, ammonia and oxygen react to form nitric acid (HNO3) and water:
NH₂(g) + 20₂(g)
HNO3(g) + H₂O(g)
1
Suppose the yield of the first step is 61.% and the yield of the second step is 60.%. Calculate the mass of hydrogen required to make 2.0 kg of nitric acid.
Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.