General Questions and Answers

Physical Chemistry
GeneralThe fluorine-17 nuclide radioactively decays by electron capture. Write a balanced nuclear chemical equation that describes this process.

Physical Chemistry
GeneralSuppose a hydrogen-2 nuclide transforms into a helium-3 nuclide by absorbing a proton.
Complete the nuclear chemical equation below so that it describes this nuclear reaction.
S
Ed
TE

Physical Chemistry
GeneralSuppose a carbon-14 nuclide decays into a nitrogen-14 nuclide by emitting an electron.
Complete the nuclear chemical equation below so that it describes this nuclear reaction.
14
ỐC - D
X
Y
5
?

Physical Chemistry
GeneralSuppose a plutonium-239 nuclide transforms into a curium-242 nuclide by absorbing an alpha particle and emitting a neutron,
Complete the nuclear chemical equation below so that it describes this nuclear reaction.
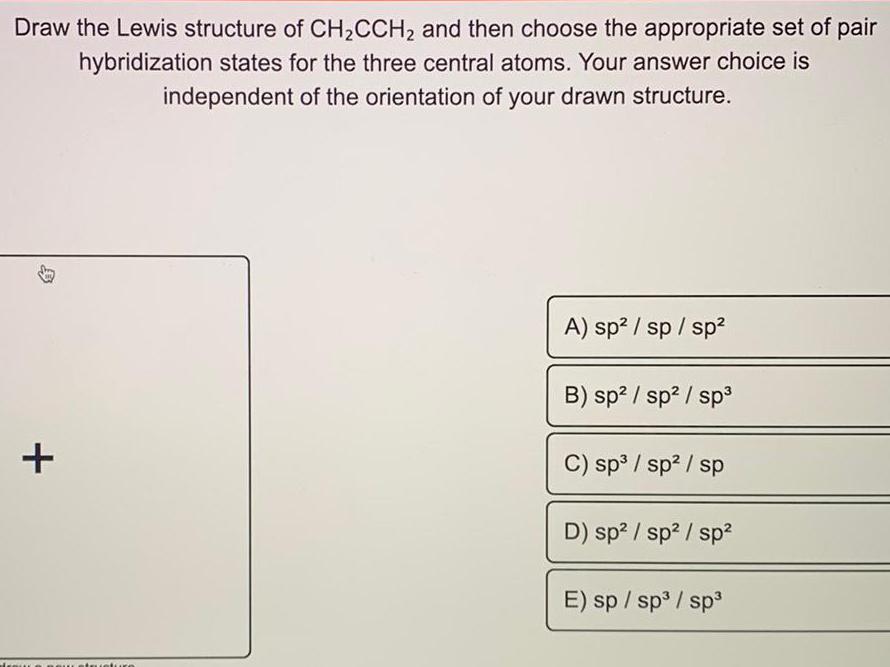
Physical Chemistry
GeneralDraw the Lewis structure of CH₂CCH₂ and then choose the appropriate set of pair
hybridization states for the three central atoms. Your answer choice is
of the orientation of your drawn structure.
independent
+
A) sp² / sp / sp²
B) sp² / sp² / sp³
C) sp³ / sp² / sp
D) sp² / sp² / sp²
E) sp / sp³ / sp³

Physical Chemistry
GeneralWhich option contains a sentence that uses quotation marks correctly?
"Are you going to take the test tomorrow"? she asked.
O "Are you going to take the test tomorrow? she asked".
O "Are you going to take the test tomorrow?" she asked.
All of the choices

Physical Chemistry
GeneralSuppose the amount of a certain radioactive substance in a sample decays from 1.10 mg to 900. µg over a period of 16.0 years. Calculate the half life of the
substance.
Round your answer to 2 significant digits.

Physical Chemistry
GeneralAn atom consists of a small, positively charged
nucleus, surrounded by negatively charged
electrons. We organize the electrons in a logical
manner. As the atomic number increases, electrons
are added to the subshells according to their
energy. Lower energy subshells fill before higher
energy subshells. The order of filling is 1s, 2s, 2p,
3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f,
6d, 7p.
The periodic table can be used to help you
remember the order.
Part A
Review Constants I Periodic Table
Give the complete ground-state electron configuration for silicon (Si).
Express the complete electron configuration using superscripts where appropriate. For example, the
configuration for Li would be entered as 1s^22s^1.
▸ View Available Hint(s)
1s^2, 2s^2, 2p^6, 3s^2, 3p^2.

Physical Chemistry
Generalthe Review link to access the section in your
e Text.
Identify the solute and solvent in each of solution.
sugar water
water is solvent and sugar is solute
sugar is solvent and water is solute
Submit
▾ Part C
soda water
Request Answer
carbon dioxide is solvent and water is solute
water is solvent and carbon dioxide is solute

Physical Chemistry
General3. Ozone, a component of smog, is a toxic substance that consists of only oxygen atoms. If
ozone has a density of 2.1 g/L at standard conditions, then what is the chemical formula of
ozone?
Hint: Determine the molar mass of ozone.

Physical Chemistry
GeneralThe mercury-201 nuclide radioactively decays by electron capture. Write a balanced nuclear chemical equation that describes this process.

Physical Chemistry
GeneralSuppose a fluorine-17 nuclide transforms into an oxygen-17 nuclide by absorbing an electron and emitting a gamma ray.
Complete the nuclear chemical equation below so that it describes this nuclear reaction.

Physical Chemistry
GeneralA sealed container holding 0.0255 L of an ideal gas at 0.979 atm and 75 °C is placed into a refrigerator and cooled to 43 °C with
no change in volume. Calculate the final pressure of the gas.
P =
atm

Physical Chemistry
GeneralA 15-m-tall barrel is closed on top except for a
thin pipe extending 4.8 m up from the top. When
the barrel is filled with water up to the base of the
pipe (1.5 m deep) the water pressure on the
bottom of the barrel is 15 kPa.
Part A
What is the pressure on the bottom when water is added to fill the pipe to its top?
Express your answer to two significant figures and include the appropriate units.

Physical Chemistry
GeneralChoose the sentence which uses the apostrophe correctly.
O I went to my father-in-law house' for dinner last week.
I went to my father-in-laws house for dinner last week.
O I went to my father-in-law's house for dinner last week.

Physical Chemistry
GeneralThe following reaction is used to make carbon tetrachloride, CCl4, a solvent and
starting material for the manufacture of fluorocarbon refrigerants and aerosol
propellants.
CS₂(g) + 3Cl₂(g) ----> CCl4(g) + S₂Cl₂(g)
Calculate the number of grams of carbon disulfide, CS2, needed for a laboratory-
scale reaction with 66.70 g of chlorine, Cl₂?
Express your answer to 2 decimal places.

Physical Chemistry
GeneralUse the following information to determine the lattice energy (in kJ/mol) of KCI(s):
lonization energy of K(g) = 418.7
Enthalpy of sublimation of K(s) = 89.2
Enthalpy of formation of KCI(s): -435.9
Electron affinity of Cl(g) = -349.0
Bond energy of Cl₂(g) = 240.0

Physical Chemistry
GeneralIn May 2016, William Trubridge broke the world record in free diving (diving underwater without the use of supplemental
oxygen) by diving to a depth of 124 m. Assume that he takes a breath that fills his lungs to 3.6 L at the surface of the water
(1.0 atm). Calculate the volume that this same amount of air will occupy in his lungs when he reaches a depth of 124 m
(13.3 atm). Enter the answer in units of liters.

Physical Chemistry
GeneralWhich of the following statements is true?
Multiple Choice
A molecule that is superimposable on its mirror image is chiral.
A chiral molecule usually contains a plane of symmetry.
An achiral molecule does not contain a plane of symmetry.
Enantiomers are mirror images that are not superimposable.

Physical Chemistry
GeneralMix-'n'-match! Match the statements on the left with the statements on the right. You should use each of
the statements on the right only once.
AE> 0
AE < 0
q>0
9 <0
W> 0
W<0
A. heat is transferred from the system to the surroundings
B. work is done by the surroundings on the system
C. energy is transferred from the system to the surroundings
D. work is done by the system on the surroundings
E. energy is transferred from the surroundings to the system
F. heat is transferred from the surroundings to the system

Physical Chemistry
GeneralSelect all that apply.
5 points
Which of the following statements are true for batteries? Select all that apply.
A.
A fuel cell is an electrochemical cell but not a galvanic cell.
B.
A fuel cell requires no reactants other than the contents of the fuel cell.
C.
A battery is an electrochemical cell but not a galvanic cell.
D.
Lithium batteries provide a large current and are lighter than comparable batteries of other types.
Lithium ion batteries are used in many portable electronic devices, including all-electric vehicles.
E.

Physical Chemistry
GeneralWhich of the following is the definition of chirality?
Multiple Choice
The existence of a molecule with a carbon atom that has four different substituents.
The existence of a molecule that is not superimposable on its mirror image.
The existence of a molecule with a mirror image.
The existence of a molecule that is superimposable on its mirror image.

Physical Chemistry
GeneralCr(OH)a(s) + CIO, (aq) → CrO2 (aq) + CT (ag)
Fill in the coefficients and substances for the balanced overall equation.
Note: Do NOT leave any coeffient fields empty. If the coefficient is 1, choose "1" as opposed to
leaving it blank.
- Cr(OH), +- CIO,Co+a+

Physical Chemistry
GeneralExercise C Calculating volume/volume % Concentration (1 pt per answer, 10 total)
Complete the following table by calculating the volume/volume % for the following solutions.
The volume/volume % is the volume of the solute in mL divided by the volume of the solution in
mL x 100. The volume of the solution is equal to the volume solvent plus the volume of solute.
Fill in all of the empty spaces. The volume of the solute and the solvent must be in the same
units.
Volume solute
85.0 mL
75 mL
200. mL
64 mL
Volume solvent
175mL
4
1.200 Liters
Volume of solution
300. mL
750. mL
Volume/volume %
34.%
45%

Physical Chemistry
GeneralNi(CH3CO₂)2 + K₂SNIS + KCH3CO₂
The above reaction between nickel(II) acetate and potassium sulfide produces nickel(II) sulfide and
potassium acetate. Balance the reaction. What is the coefficient in front of potassium acetate (KCH3CO₂)
once balanced?

Physical Chemistry
GeneralLook at your model and think about the relationship between oxygen and carbon dioxide in the sealed jar. Which of these statements is true?
Plants and animals release oxygen during respiration.
O Plants release only carbon dioxide during photosynthesis.
Plants use oxygen during photosynthesis.
Plants and animals use oxygen during respiration.

Physical Chemistry
GeneralCalculate the cell potential at 25°C for the
Cr(s) Cr³+ (aq)||Sn²+ (aq)|Sn(s)
electrochemical cell if the concentration of Cr³+ (aq) is 0.50 M and the concentration of Sn²+ (aq) is 0.090 M. Report
your answer to 3 significant figures.
E
cell
Type numbers in the boxes
10 points
V

Physical Chemistry
GeneralIdentify the precipitate(s) of the reaction that occurs when a silver nitrate solution is mixed with a sodium chloride solution.
Check all that apply.
View Available Hint(s)
sodium nitrate
silver nitrate
silver chloride
sodium chloride

Physical Chemistry
GeneralWhat is the insoluble solid substance that is produced during some aqueous chemical reactions? In this
reaction example, that would be AgF(s).
NaF(aq) + AgNO3(aq) → AgF(s) + NaNO3(aq)
Osolute
O precipitate
Otitrant
Oreactant
O empirical formula

Physical Chemistry
GeneralWhich one of the following statements is true? Select all that apply.
A. An electrolytic cell does not require external voltage to operate.
B.Oxidation occurs at the anode in a galvanic cell but not in an electrolytic cell.
C. Reduction occurs at the cathode in a galvanic cell.
D. When a galvanic cell is recharged, the reverse chemical reaction occurs as when the galvanic cell operates.
E.Oxidation occurs at the cathode in an electrolytic cell.
Select all that apply.
5 points

Physical Chemistry
GeneralEnter your answer in the box provided.
What is the ee for the following mixture of enantiomers A and B?
90% A and 10% B
% ee

Physical Chemistry
GeneralThe following information is given for chromium at 1 atm:
Tb = 2672.00°C
Tm=1857.00°C
Use the References to access important values if needed for this question.
2022.00°C.
AHvap (2672.00°C) = 5.874 × 10³ J/g
X
AHfus (1857.00°C) = 281.5 J/g
Specific heat solid = 0.4600 J/g °C
Specific heat liquid = 0.9370 J/g °C
A 35.20 g sample of solid chromium is initially at 1827.00°C. If the sample is heated at constant pressure (P = 1 atm),
kJ of heat are needed to raise the temperature of the sample to

Physical Chemistry
GeneralA brass alloy contains 70.1% copper, Cu, by mass with the remainder of the alloy being zinc, Zn. Determine the number of
moles of zinc in 45.5 g of the brass alloy.
moles of Zn =
13.6
atoms of Zn =
Incorrect
A
Determine the number of atoms of zinc in 45.5 g of the brass alloy.
3.02 ×1023

Physical Chemistry
GeneralHow many molecules are present in each sample?
4.75 mol SO₂:
0.603 mol CO:
85.5 x1023
Incorrect
7.236 ×1023
Incorrect
a sample of NH3 containing Avogadro's number of molecules:
24 x1023
molecules SO₂
molecules CO
molecules NH3

Physical Chemistry
GeneralWhich of the following statements about constitutional isomers is not true?
Multiple Choice
They have different IUPAC names.
They have different physical properties.
They always have the same functional groups.
They have different chemical properties.

Physical Chemistry
GeneralBelow is the structure of fructose, is it D- or L-fructose AND which numbered carbon determines
this?
CH₂OH
C=O
HO-H
H-OH
H-OH
CH₂OH
L-fructose; #4 carbon
D-fructose; # 5 carbon
D-fructose; #4 carbon
OL-fructose; #5 carbon
OL-fructose; #2 carbon
10
![For carbon-based combustion reactions, there is a recommended order (a "rule") in which the atoms should
be balanced initially. Put the atoms in the correct order for balancing according to this rule.
should be balanced first.
should be balanced second.
should be balanced last.
Question 10
[Choose]
[Choose ]
sodium
nitrogen
carbon
calcium
helium
oxygen
hydrogen
1 pts](https://media.kunduz.com/media/sug-question/raw/58760782-1659704659.9239933.jpeg?w=256)
Physical Chemistry
GeneralFor carbon-based combustion reactions, there is a recommended order (a "rule") in which the atoms should
be balanced initially. Put the atoms in the correct order for balancing according to this rule.
should be balanced first.
should be balanced second.
should be balanced last.
Question 10
[Choose]
[Choose ]
sodium
nitrogen
carbon
calcium
helium
oxygen
hydrogen
1 pts

Physical Chemistry
GeneralYou add 100.0 g of water at 55.1 °C to 100.0 g of ice at 0.00 °C. Some of the ice melts and cools the water to 0.00 °C. When the ice and water mixture reaches thermal equilibrium at 0 °C, how much ice has
melted? (The specific heat capacity of liquid water is 4.184 J/g. K. The enthalpy of fusion of ice at 0 °C is 333 J/g.)
Mass of ice =
g

Physical Chemistry
GeneralIn an experiment, the decomposition of hydrogen peroxide, H₂O2 (aq), was used to produce oxygen gas, O2 (g).
2 H₂O2 (aq)
2 H₂0 (1) + O₂ (g)
The reaction flask contained the H₂O2 and the test tube inside the flask contained a catalyst to speed up the reaction. The volume of oxygen gas produced
was determined by water displacement. To determine the moles of oxygen produced, the reaction flask, including its contents, was weighed before and after
the reaction. During the reaction the flask was placed in a cold water bath to cool the solution. After the reaction, if the outside of the reaction flask was still
wet when it was weighed, how would this affect the molar volume of the oxygen gas that was determined?
->
Select one:
OA. The molar volume of the oxygen gas determined would be too large.
B. More information is needed to answer this question.
O C. This would not significantly affect the molar volume of the gas.
O D. The molar volume of the oxygen gas determined would be too small.

Physical Chemistry
GeneralWhich of the following statements about meso compounds is not true?
Multiple Choice
A meso compound and its mirror image are identical.
A meso compound generally has a plane of symmetry.
A meso compound is chiral.
A meso compound is achiral.

Physical Chemistry
GeneralRank the following groups in order of decreasing priority according to the Cahn-Ingold-Prelog system.
I>II> IV > III
III > II > IV>I
III>IV>II>I
III> I >II>IV

Physical Chemistry
GeneralDetermine the volume (mL) required to prepare
each of the following.
▼
Part A
18.0 mL of a 0.200 M KNO3 solution from a 4.00 M KNO3 solution.
Express your answer with the appropriate units.
Value
Submit
μÀ
Part B
→
Units
Request Answer
?
20.0 mL of 2.50 M H₂SO4 solution using a 12.5 M H₂SO4 solution.
Express your answer with the appropriate units.
Review | Cons

Physical Chemistry
GeneralSelect one answer
5 points
Which of the following descriptions is true for the cell below?
Zn(s) | Zn²+ (aq) (0.5 M) || Cu²+ (aq) (1.0 M) | Cu(s)
A. O When the cell reaches equilibrium, E = 0, and the cell will no longer work.
cell
B. O At 25 °C, when the reaction quotient, Q, is equal to the equilibrium constant, K, Eºcell = o.
C. When the reaction quotient, Q, changes from its original value to when it equals 1, E does not change
D. OE can be determined by using the standard half-cell potentials alone.

Physical Chemistry
GeneralAnswer the following for the reaction:
Mg(s) + 2HCl(aq)-H₂(g) + MgCl₂ (aq)
How many liters of hydrogen gas can form at STP when 0.491 L of a 2.02 M HCl solution reacts with excess magnesium?
Express your answer with the appropriate units.
What is the molarity of a HCl solution if the reaction of 47.6 mL of the HCl solution with excess magnesium produces 5.20 L of H₂ gas at 735 mmHg and 25 °C?
Express your answer with the appropriate units.

Physical Chemistry
GeneralIdentify the correct in-text citation based on APA style for this source:
Tannen, D. (1998). The argument culture. Toronto: Random House.
Every issue we see discussed on television appears to be set up as an argument: "In the
O argument culture, criticism, attack, or opposition are predominant if not the only ways of
responding to people or ideas" (Tannen, 1998, p. 7).
Every issue we see discussed on television appears to be set up as an argument: "In the
argument culture, criticism, attack, or opposition are predominant if not the only ways of
responding to people or ideas" (p. 7).
Every issue we see discussed on television appears to be set up as an argument: "In the
argument culture, criticism, attack, or opposition are predominant if not the only ways of
responding to people or ideas" (The argument culture, 1998, p. 7).

Physical Chemistry
GeneralDraw the Lewis structure of CH3NCO and then choose the appropriate set of
hybridization states for the three central atoms. Your answer choice is
independent of the orientation of your drawn structure.

Physical Chemistry
GeneralIf the energy of the system is the sum of the energy and work, identify the following: What happens to the volume if work was done
by the system?
A Volume increases
B
Volume decreases

Physical Chemistry
GeneralConvert 0.3330 mol of Na₂SO4 to grams. Show the unit analysis by inserting the correct components into the unit-factor slots. It may be useful to consult the periodic table.
0.3330 mol Na₂SO4 X

Physical Chemistry
GeneralConsider the chemical reaction that occurs when 50.00
g of dinitrogen tetroxide (N₂O4, 92.02 g/mol) reacts
with 47.50 g of hydrazine (N₂H4, 32.06 g/mol) to
produce nitrogen gas and water.
How many grams of excess reagent are left over after
the reaction has run to completion if the percent yield
of the reaction was 100%?
Excess reagent left over after the reaction with 100%

Physical Chemistry
GeneralWhat did MITS design focus on?
Increasing weight to minimize the energy needed to maintain height.
Reducing the weight to minimize the energy needed to maintain height.
Increasing drag to reduce the loss of energy to the air.
Minimizing drag to reduce the loss of energy to the air.