General Questions and Answers

Physical Chemistry
GeneralI If 2 4 gm of a metal displace 1 12 litre hydrogen at normal temperature and pressure Equivalent weight metal would be 1 12 4 1 2 11 2 2 24 3 1 2 x 11 2

Physical Chemistry
GeneralAn intensive property of a substance is O a independent of the amount present Ob dependent only on its temperature Oc d Oe not affected by its temperature dependent on its volume but not its mass dependent only on its mass and volume
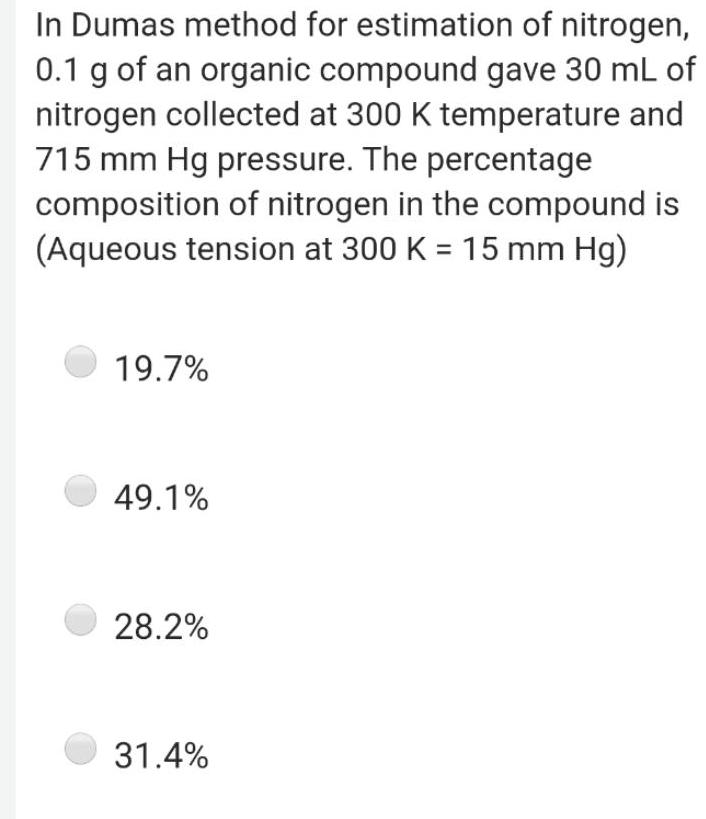
Physical Chemistry
GeneralIn Dumas method for estimation of nitrogen 0 1 g of an organic compound gave 30 mL of nitrogen collected at 300 K temperature and 715 mm Hg pressure The percentage composition of nitrogen in the compound is Aqueous tension at 300 K 15 mm Hg 19 7 49 1 28 2 31 4

Physical Chemistry
General5 Rearrange the following I to IV in the order of increasing masses and choose the correct answer Atomic masses N 14 O 16 Cu 63 1 molecule of oxygen I II 1 atom of Nitrogen III 1 x 10 10 x gm molecular weight of oxygen IV 1 x 10 10 x gm atomic weight of copper 1 II I III IV 2 IV III II I 3 II III I IV 4 III IV I II

Physical Chemistry
General1 A solution contains 1 44 10 2 M potassium chromate and 5 58 10 3 M potassium sulfate Solid lead nitrate is added slowly to this mixture What is the concentration of chromate ion when sulfate ion begins to precipitate chromate M

Physical Chemistry
Generalc less than antistokes lines d equal to Rayleigh line 20 Homonuclear diatomic molecules are often found to be a microwave active b IR active c uv active d Raman active O 2 State True or False

Physical Chemistry
GeneralA compound of magnesium contains 21 9 magnesium 27 8 phosphorus and 50 3 oxygen What will be the simplest formula of the compound a Mg P O c Mg P 0 3 b MgPO d MgP 04 1 V in

Physical Chemistry
GeneralA 0 1064 g sample of a pesticide was decomposed by the action of sodium biphenyl in toluene The liberated CI was extracted with water and titrated with 23 28 mL of 0 03337 M AgNO3 using an adsorption indicator Express the results of the analysis in terms of percent aldrin C12H8C16 FW 364 92

Physical Chemistry
GeneralA giant molecule contains 0 25 of a metal whose atomic weight is 59 Its molecule contains one atom of tha metal Its minimum molecular weight is 1 5900 2 23600 3 11800 4 100 59

Physical Chemistry
GeneralWrite antonyms of the underlined words and rewrite the sentences i Some clothes were damp when I went to the backyard Ans ii The leaves of the mango tree look dirty Ans iii The children were happy to see the stranger

Physical Chemistry
GeneralA metal M reacts with dil H SO4 is present as its metal sulphide X On heating X in air gives oxide Y and a gas Z X Y Z are respectively copper sulphide copper oxide sulpur dioxide O zinc sulphide zinc oxide sulpur dioxide O copper sulphide copper oxide carbon dioxide O zinc sulphide zinc oxide carbon dioxide

Physical Chemistry
General30 A B and C are in equilibrium as shown in the diagram Which of the following relations among the rat constants is true B A K k3 K 3 2 K2 A k k k 3 k3k 1k 2 B k k k3 k 3k 1k 2 C k k 2k3 k 3k 1k D k 1k2k3 k 3k k 2 C

Physical Chemistry
GeneralBalance the following reactions a Mg N Mg3N2 b PCL5 H O H3PO4 HCe c d e f h i Al Fe3O4 Al O3 Fe MnO HCl MnCl Cl2 H O Na S O3 12 Nal NaS O Sn NaOH Na SnO H P4O10 H2O H3PO4 Al2 SO4 3 NaOH Al OH 3 Na SO4 CH3OH O2 CO H O Ammonium chloride and barium hydroxide is heated and the compounds react to ammonium gas barium choride and water Remember to include phase labels

Physical Chemistry
GeneralWhich of them is correct statement O Cu can be recovered by CuSO4 by use of Zn O Zn can be recovered by ZnSO4 by use of Ag O Cu can be recovered by CuSO by use of Ac O Zn can be recovered by ZnSO4 by use of Cu

Physical Chemistry
GeneralWhich of the following pairs of a chemical reaction certain to result in a spontaneous reaction 1 Endothermic and decreasing disorder 2 Exothermic and increasing disorder 3 Endothermic and increasing disorder 4 Exothermic and decreasing disorder

Physical Chemistry
GeneralCollect the following solids grannular sugar common salt blue vitriol Observe a few grannules of these solids under a magnifying lens or microscope Discuss your observations with reference to the following points i Shape of the grannules ii Smoothness of faces of the grannules and iii Angles between various edges of the grannules All the above solids are crystalline solids Name the properties of crystals that you observed in this activity SALT

Physical Chemistry
General6 The following equilibrium constants are given N 3H 2NH3 K N O22NO K H 0 H O K The equilibrium constant for the oxidation of NH3 by oxygen to give NO is a K K3 K K K b K3 K3 K K K

Physical Chemistry
GeneralAn ideal solution was found to have a vapour pressure of 80 torr when the mole fraction of a non volatile solute was 0 2 What would be the vapour pressure of the pure solvent at the same temperature Question Type Single Correct Type 1 2 3 64 torr 80 torr 100 torr 100 torr

Physical Chemistry
General5 Ag O H O 2e2Ag 2OH In the above chemical reaction 1 Water is oxidised 2 Silver is oxidised 3 Silver is reduced 4 Hydrogen is reduced imited Regd Of

Physical Chemistry
GeneralElectrons in a sample of H atoms make transition from state n x to some lower excited state The emission spectrum from the sample is found to contain only the lines belonging to a particular series If one of the maximum energy photons has an energy of 0 6375 eV find the value of x 3 Take 0 6375 eV 0 85 eV 4

Physical Chemistry
General29 Which of the following statements is are true 1 Point An oil can be pumped easily below cloud point An oil can be pumped easily above cloud point An oil can be pumped easily below pour point

Physical Chemistry
GeneralContainer Gas Formula Molar mass g mol Temperature C Pressure atm B Ethane CHA C H6 A Methane 16 27 2 0 30 e density of the gas in g L is Ogreatest in container A O greatest in container B Ogreatest in container C the same in all three containers 27 e average kinetic energy of the gas molecules is Ogreatest in container A O greatest in container B O greatest in container C the same in all three containers 4 0 the pressure of each gas is increased at constant nperature until condensation occurs which gas Il condense at the lowest pressure Methane Ethane O Butane O All the gases will condense at the same pressure Butane C4H10 58 27 2 0

Physical Chemistry
GeneralAn acid is a component of rancid butter and has a vile stench Burning 0 44 g of the acid in excess oxygen yield 0 88 g o f CO2 and 0 36 g of H20 as the only pro ducts Given that the acid contains only C H and O and if vapour density of the acid is 44 then which of the following o ptions is not correct about it s molecula r formula Please explain in an easy ma nner I dont understand why one mole will be equal to 0 04mol of hydrogen

Physical Chemistry
GeneralParagraph for Q Nos 4 to 5 12 mL gaseous mixture of an alkane and an alkene contain ing same number of carbon atoms require exactly 285 mL of air containing 20 v v O and rest N for complete com bustion at 200 K After combustion when gaseous mixture is passed through KOH solution it shows volume contraction of 36 mL 4 Formula of alkane is a C5H 2 b CH c C H6 d C4H10 5 Mole fraction of CO in final gaseous sample is 6 d 4 13 a 6 b 6 13 c 6 14

Physical Chemistry
General4 For the following reaction pick out the incorrect interpretations 2A g B g 4C g D g 1 2 moles of A 1 mole of B reacts to form 4 moles of C 1 mole of D 2 2 gm of A 1 gm of B reacts to give 4 gm of C 1 gm of D 3 2 molecules of A 1 molecule of B reacts to give 4 molecules of C 1 molecule of 4 2 vol of A 1 vol of B reacts to give 4 vol of C 1 vol of D At STP

Physical Chemistry
GeneralFor each of the following determine if the first item or the second item has the larger entropy NO g OR NO2 g A Second item B First item SO3 g OR S S Ice at 40 C OR Ice at 4 C CO g or CO2 g

Physical Chemistry
GeneralSelect the incorrect statement regarding the boiling points of alkanes a boiling point increases with stronger van der Waal s forces MHT CET Chemistry b surface area is the only factor which determines the boiling point of alkane 11 00 25 12 37 An ma c a boiling point of straight chain alkanes is greater than that of branched chain alkanes c d the boiling point of cycloalkanes is always 38 Alk

Physical Chemistry
GeneralThe molality of 1 litre solution with y w v of CaCO is 2 The weight of the solvent present in the solution is 450 g then value of y is At wt Ca 40 C 12 0 16

Physical Chemistry
GeneralA chemist isolated a protein from crab and wanted to determine its molecular weight Which of the following method would give the best result for him A Elevation in boiling point B Relative lowering of vapour pressure C Depression in freezing point D Osmotic pressure method

Physical Chemistry
General72 Pure PCl is introduced into an evacuated chamber and comes to equilibrium at 247 C and 2 0 atm The equilibrium gaseous mixture contains 40 chlorine by volume Calculate K at 247 C for the reaction a 0 625 atm c 1 6 atm PC15 g PC13 g Cl g b 4 atm d None of these

Physical Chemistry
GeneralConcentration of the Ag ions in a saturated solution of Ag C O4 is 2 2 x 10 mol L 1 Solubility product of Ag C A 2 42 x 10 8 C 4 5 x 10 11 01 B 2 66 x 10 12 D 5 3 10 2

Physical Chemistry
Generala microwave active b IR inactive c microwave inactive d none of these 14 The selection rule for rigid diatomic molecule for rotational transitions describes d AJ 1 a Av 0 b Av 1 c AJ 0 15 The zero point energy of molecule is given as

Physical Chemistry
General1 C B D A Y2 A C B D 3 B D A In context with the industrial preparation of hydrogen from water gas CO H which of the following is the correct statement AIEEE 2008 1 CO is removed by absorption in aqueous Cu Cl Solution 2 H is removed through occlusion with Pd 3 CO is oxidized to CO with steam in the presence of a catalyst followed by absorption of CO in alkali 4 CO and H are fractionally separated using differences in their densities 2 is statements is incorrect regarding physissorptions AIEEE 2009

Physical Chemistry
GeneralD K 2 Jog K Surface tension of lyophilic sals is Lower than that of H O 139 B1 2 More than that of H O 4 None of the above Habt from collidal solution the effect due to scattering of light is known as 3 Equal to that of H O try Choice A

Physical Chemistry
General6 Consider the following diagram Q 5 to 6 A Which of the following change is physical change 1 only A 2 only B B Which of the following change is chemical change 2 only B 1 only A 3 A and B both DO 3 A and B both 8 4 None of A and B 4 None of A and B

Physical Chemistry
Generalc polarizability d dipole moment The plot of total molar polarization PT vs inverse of absolute temperature 1 T of the system in case of carbon tetrachlloride is a parallel to 1 T axis b parallel to PT axis c passing through origin d with positive intercept on Praxis Expooted value of dingle moment in Debye units in case of p dichlorobenzene is

Physical Chemistry
GeneralA metal M which is not affected by strong acids like conc HNO3 conc H SO4 and concentrated solutions of alkalies like KOH and NaOH but dissolves in aqua regia and forms MCI3 which is used for toning in photography The metal M is O Ag OHg O Au CUL

Physical Chemistry
GeneralPh The rate law for the following intramolecular Cannizzaro reaction will be i i H a rate k Ph c rate K Ph OH 1 8 8 Ph CH ph i i HON H OH www i HITOH d rate k Ph C C H OH b rate K Ph C H OH

Physical Chemistry
GeneralQ 10 SINGL The voltage of the cell consisting of Li s and F2 g electrodes is 5 92 V at standard condition at 298 K What is the voltage if the electrolyte consists of 2 M LiF In2 0 693 R 8 314 JK mol and F 1 B C 5 90 V 5 937 V 5 88 V Correct Answer 4 9 V 96500C mol

Physical Chemistry
GeneralAs per Valence Bond Theory V B T which of the following overlapping is are possible Od dy 0 Py F sp on X axis common axis Px Py sp on X axis common axis on X axis common axis on X axis common axis

Physical Chemistry
General1 1 2 1023 3 10 AIEEE 2006 In langmuir s model of adsorption of a gas on a solid surface 1 the rate of dissociation of adsorbed molecules from the surface does not depend on the surface covered 0 Um 2 the adsorption at a single site on the surface may involve multiple molecules at the same time 3 the mass of gas striking a given area of surface is proportional to the pressure of the gas 4 the mass of gas striking a given area of surface is independent of the pressure of the gas illoide B Cand D are 0 50 0 01 0 10 and 0 005 respectively The correct IEEE 20081

Physical Chemistry
GeneralThe specific conductance of weak acid HA whose concentration 0 1 M is 0 001 ohm 1 cm 1 Its molar conductance is 0 001 0 01 1 0 10 Marks 4 1

Physical Chemistry
GeneralFor non polar molecules the molar polarization is a deformability c polarizability b induced molar polarization d dipole moment 0 of absolute temperature

Physical Chemistry
GeneralFor the two reactions I AB II C D following graph is obtained conca IMCI 0 5M A 30 for 60 Time min Which of the following is true If B A then at that time B D If C A then at that time B D t100 Reaction 1 t100 Reaction II 3 2 3 A C at t min M

Physical Chemistry
GeneralMass of KHC O potassium acid oxalate required to reduce 100 mL of 0 02 M KMnO in acidic medium to Mn is x g and to neutralise 100 mL of 0 05 M Ca OH is y g then B 2x y A x y C x 2y D None is Correct SPACE FOR ROUGH WORK

Physical Chemistry
GeneralEfficiency of a cell with cell reaction under standard conditions A s B A B s AH 300kJ is 70 The standard electrode potential of cell is O 2 176 V O 2 876 V O 1 248 V 1 648 V

Physical Chemistry
GeneralAt a given temperature in order for a reaction o be favored spontaneous the reaction should have a relatively A Small AH and a small AS B Large AH and a large AS C Small AH and a large AS D Large AH and a small AS

Physical Chemistry
GeneralAll stion No 6 moles of gas compressed reversible from 10 L to 1 L at constant temperature The entropy change during the process is 10 R D 10 R Physics 23 03 R O 23 03 R CP

Physical Chemistry
GeneralSuppose 5 272 g of a soil sample undergoes an extraction with 50 mL of extracting solvent to remove the potassium This 50 mL is then diluted to 250 mL and tested with an instrument The concentration of potassium in this diluted extract is found to be 35 7 ppm What is the potassium concentration in parts per million in the untreated soil sample O a 1000mg kg O b 125ppm O c 1692 90mg kg O d 2090mg kg

Physical Chemistry
General0 4 Sulphuryl chloride SO Cl reacts with H O to give mixture of H SO and HCl Aq solution of one mol SO Cl will be neutralised by 4 A 3 mol of NaOH B 2 mol of Ca OH C both D None O A OB O C