General Questions and Answers

Physical Chemistry
GeneralSO3 can be prepared by the following sequence of reactions Sg 8 02 8 SO2 50 yield Reaction I 2 SO2 02 2 SO3 100 yield Reaction II A sample containing 50 by mass of each Sg and O2 is taken in the initial reaction mixture If the sum of weights of reactants initially taken to obtain 320 g of SO3 is x g for reaction I then value of is X 16 yield of reactions are mentioned

Physical Chemistry
GeneralThe spinel structure consists of an array of 0 ions in fcc arrangement General formula of spinel is AB 04 Cations of A occupy 1 8 th of the tetrahedral voids and cations of B occupy half of the octahedral voids Spinels are always electrically neutral and these are important class of compounds There are also inverse spinels in which the distribution is B AB 04 Those in square brackets denote the species in octahedral voids Question 18 Which of the following is an inverse spinel Options a PbFe 04 b ZnO Al O3 c Fe304 d Mg0 Al O3

Physical Chemistry
GeneralA mixture of C3H8 and oxygen in 1 L closed vessel has an internal pressure of 4 atm at 100 C When the mixture is ignited the reaction produces CO g and H O g until all the oxygen is consumed After the reaction pressure of the vessel is 4 2 atm at the same temperature The mass of oxygen present before the reaction is L atm mol K R 0 082

Physical Chemistry
GeneralWhen crystals of potassium chloride is heated with Mn2O3 mixedoxide and conc H SO4 then ID Q 529182 Options are A Cl is produced B Its redox change C Resulting mixture after dilution by water gives buff colour ppt with H S D Resulting mixture after dilution by water gives ppt with excess KOH

Physical Chemistry
Generals p t Cs D q t 44 How many moles of ferric alum NH SO Fe SO 24H O can be made from the sample of Fe containing 0 0056 g of it a 10 mol b 0 5 x 10 mol c 0 33 10 mol d 2 104 mol

Physical Chemistry
General36 3 68 of a mixture of CaCO and MgCO is heated to liberate 0 04 mole of CO The mole of CaCO and MgCO in the mixture is respectively a 50 50 b 60 40 c 40 60 d 30 70

Physical Chemistry
General5 Assertion A certain element X forms three binary compounds with Chlorine containing 59 68 68 95 and 74 75 Chlorine respectively These data illustrate the law of multiple proportions Reason According to law of multiple proportions the relative amounts of an element combining with some fixed amount of a second element in a series of compounds are the ratios of small whole numbers

Physical Chemistry
Generald 6 63 x 10 2 m P 43 If E E and E represents the kinetic energy of an electron a particle and proton respectively each moving with same de broglie wavelength then a E E E e P c E E E P a a b E E E d E E E P e a

Physical Chemistry
Generala 66 6 c 25 b 75 d 33 3 38 If 42 g of an unknown gas X occupies a volume of 125 L at 0 3 bar pressure and 300 K temperature then the gas X could be a N C CO b CO d NO 45

Physical Chemistry
General11 Which of the following statements about a compound is incorrect a A molecule of a compound has atoms of different elements b A compound cannot be separated into its constituent elements by physical methods of separation c A compound retains the physical properties of its constituent elements d The ratio of atoms of different elements in a compound is fixed

Physical Chemistry
GeneralTwo radioactive nuclides A and B have half life 100 min and 50 min respectively A fresh sample contains nuclides of B to be 16 times that of A The time required by the nuclide A to become 4 times that of B is x hours Find the value of X lin 5

Physical Chemistry
GeneralIn the estimation of sulphur organic compound on treating with conc HNO is converted to 1 SO 2 H S 3 H SO 4 SO Calculate the number of atoms in each of the following 57 moles of Ar in 57 of he iii 52 g of He NA 6022 10

Physical Chemistry
General5 A 25 0 mm x 40 0 mm piece of gold foil is 0 25 mm thick The density of gold is 19 32 g cm How many gold atoms are in the sheet Atomic weight Au 197 0 A 7 7 x 10 3 C 4 3 10 1 B 1 5 x 1023 D 1 47 x 10

Physical Chemistry
GeneralWhich of the following properties don t help in differentiating different hydrated isomers of CrCl 6H 0 1 Conductivity measurements 3 Dipole moment 2 Precipitation by AgNO 4 Magnetic moment

Physical Chemistry
General4 The number of atoms present in one mole of an element is equal to Avogadro number Which of the following element contains the greatest number of atoms a 4 g He b 46 g Na c 0 40 g Ca d 12 g He 5 If the

Physical Chemistry
General23 1 g sample of alkaline earth metal react completely with 4 08 g H SO and yields an ionic product MSO Then find out the atomic mass of alkaline earth metal M a 9 b 24 c 40 d 87 32

Physical Chemistry
Generalmich of the following molecules would have the following potential energy diagramfor rotation about its C2 C3 bon Energy kcal mol 0 0 9 180 butane 5 0 1 8 2 methylbutane 2 3 dimethylbutane 5 0 O torsional angle 0 9 60 4 2 120 09 180

Physical Chemistry
GeneralA tetra atomic molecule X on reaction with nitrogen oxide Oxidation State 1 produces two substances Y and Z y is a dehydrating agent while compound Z is a diatomic gas which shows almost inert gas behavior The substances X Y and Z are 1 P N O O 2 P P O Ar 3 P PO O 4 P P O N Arrange the following structure according to their increasing order of acidic behavior in nolar solvent
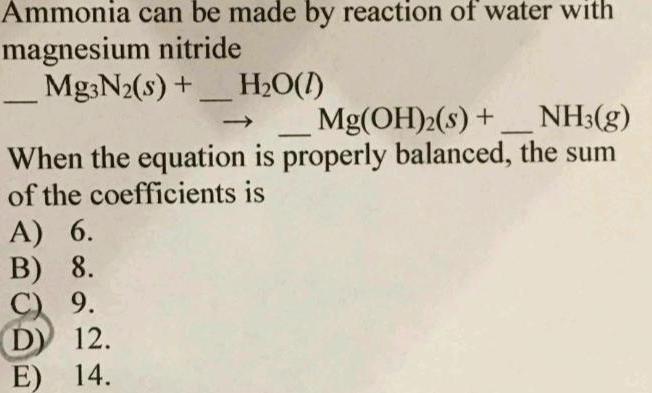
Physical Chemistry
GeneralAmmonia can be made by reaction of water with magnesium nitride Mg3N s H O 1 Mg OH s NH3 g When the equation is properly balanced the sum of the coefficients is A 6 B 8 C 9 D 12 E 14

Physical Chemistry
GeneralThe products of the following I and II sequences are related as H3C C C CH3 A Diastereomers II 1 H Pd BaSO4 2 Br CCl4 1 Br CCl4 1 eq 2 H Pd B Identical C Enantiomers D Geometrical is

Physical Chemistry
General1 18 4 A salt M4 ionizes as MAM 2A It was found that a given solution of the salt had the same freezing point as solution of glucose of twice the molality The apparent degree of ionization of the salt is 2 0 33 3 0 50 2 9 2 3 4 6 4 2 3 1 0 25 4 0 67 The solubility product of declis 18x10 19 Precenitation of doct will occur only when equa

Physical Chemistry
GeneralA certain half reaction has a standard reduction potential Ered 1 12 V An engineer proposes using this half reaction at the anode of a galvanic cell that must provide at least 1 50 V of electrica

Physical Chemistry
GeneralConsider the phase diagram shown What is the normal freezing point Pressure not to scale 72 9 atm 5 1 atm atm 31 C 0 C SOLID 78 5 C 100 C 56 7 C LIQUID GAS 78 5 C 56 7 C Temperature not to scale 31 C

Physical Chemistry
General276 62 If an electron is revolving in its Bohr orbit having 73 I Bohr radius of 0 529 A then the radius of third orbit is C a 4234 nm c 4 761 b 4496 d 5125 nm re L

Physical Chemistry
Generalc 75 mL d 500 mL 9 In an organic compound of molar mass 108 g mol C H and N atoms are present in 9 1 3 5 by weight Molecular formula can be a C H N c C H N b C H N d C H N 28

Physical Chemistry
GeneralA certain solution of 1 m benzoic acid in benzene has a freezing point of 3 1 C and a normal boiling point of 82 6 C The freezing point of benzene is 5 5 C and its boiling point is 80 1 C Analyze the state of the solute benzoic acid at two temperatures and comment

Physical Chemistry
General10 A 2B 3C AB C 2 Reaction of 6 g of A 6 1023 atoms of B 0 036 mole of C yields 4 8 g of compound AB C If the atomic masses of A C are 60 80 amu respectively the atomic mass of B is a 60 amu c 90 amu L b 50 amu d 120 amu

Physical Chemistry
General62 On analysis a certain compound was found to contain iodine and oxygen in the ratio of 254 g of iodine at mass 127 and 80 g oxygen at mass 16 What is the formula of compound a IO b 1 0 d 1 0 c 1 0 63 0 5 mol of potassium ferrocyanide com to Formula

Physical Chemistry
General3 9 Calculate velocity of an electron present in the third orbit of the hydrogen atom Also calcula number of revolutions per second that this electron makes around the nucleus Velocity of electron in 3rd orbit

Physical Chemistry
Generalmon and 59 If 13 6 eV energy is required to separate a hydrogen atom into a proton and an electron then the orbital radius of electron in a hydrogen atom is a 5 3 x 10 m c 6 3 x 10 m ne gets as b 4 3 x 10 m d 7 3 x 10 m

Physical Chemistry
Generald 23 u 17 Insulin contains 3 4 sulphur by mass What will be the minimum molecular weight of insulin a 94 117 u b 1884 u c 941 u d 976 u 18 A 100 g of a sample of haemoglobin on analysis was

Physical Chemistry
Generalexcited state 57 In which of the following Bohr s orbit n a hydrogen atom emits the photons of lowest frequency a n 2 tom 1 state en 3 state b 4 tom 2 c n 4 tom 1 d n 4 ton 3 lectron and

Physical Chemistry
Generald 35 5 g 11 4 4 g of an oxide of nitrogen gives 2 24 L of nitrogen and 60 g of another oxide of nitrogen gives 22 4 L o nitrogen at S T P The data illustrates a Law of conservation of mass b Law of constant proportions c Law of multiple proportions d Law of reciprocal proportions

Physical Chemistry
GeneralFollowing questions No 3 11 are multiple choice questions carrying I mark each Read the following statements and identify incorrect statements Carbocation behave as nucleophile In homolytic bond cleavage free radical is formed c Ethoxyethane is functional group isomer of ethanol d In ethyne two sigma and two pi bonds are present X i i iv ii i ii iii ii iv iv iii iv

Physical Chemistry
General32 Out of 1 0 g dioxygen 1 0 g atomic oxygen and 1 0 g ozone the maximum number of oxygen atoms are contained in a 1 0 g of atomic oxygen b 1 0 g of ozone c 1 0 g of oxygen gas d All contain same number of atoms 33 The maximum volume at STP is occupied by 42 4

Physical Chemistry
General1 18 5 V 2 13 5 V 3 11 5 V 4 6 5 V A capacitor of capacitance 100 F is charged by connecting it to a battery of EMF 12V and internal resistance 252 The time taken before 99 of the maximum charge is stored on the capacitor 1 0 92 ms 2 0 4 ms 3 0 8 ms 4 0 1 ms
Physical Chemistry
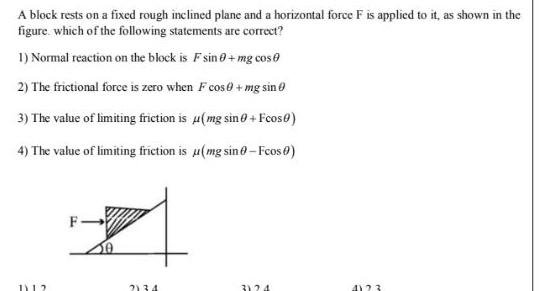
GeneralA block rests on a fixed rough inclined plane and a horizontal force F is applied to it as shown in the figure which of the following statements are correct 1 Normal reaction on the block is F sin 0 mg cos 2 The frictional force is zero when F cos mg sin 3 The value of limiting friction is u mg sin 0 Fcos0 4 The value of limiting friction is u mg sino Fcos 0 1112 F 2134 3124 4123

Physical Chemistry
GeneralPHYSICS We have three beakers A B and C containing glycerin water and kerosene respectively They an stirred vigorously and placed on a table The liquid which comes to rest at the earlest is 1 glycerin 2 water 3 kerosene 4 all of them at the same time

Physical Chemistry
GeneralIncorrect statement about PCI5 molecule is Central atom is sp d hybridised The shape of the molecule is trigonal bipyramidal Axial bonds are shorter than equatorial bonds Three P Cl bond lie in one plane

Physical Chemistry
Generalc 100 d zero The molal boiling point constant of water is 0 53 When 2 mole of glucose are dissolved in 4000 g of wa the solution will boil at a 100 53 C 100 265 C b 101 06 C d 99 47 C

Physical Chemistry
General12 If law of conservation of mass was to hold true then 20 8 g of BaCl on reaction with 9 8 g of H SO will produce 7 3 g of HCl and BaSO equal to a 11 65 g b 23 3 g c 25 5 g d 30 6 g

Physical Chemistry
General8 6 g of carbon combines with 32 g of sulphur to form CS 12 g of C also combine with 32 g oxygen to form CO 10 g of sulphur combines with 10 g of oxygen to form Sulphur dioxide Which law is illustrated by this a Law of multiple proportions b Law of constant composition c Law of reciprocal proportions d Gay Lussac s law

Physical Chemistry
Generalin a reaction N2 3H2 gives 2 NH3 40 ml of each N2 and H2 are taken to reac t together so that 25 in the of NH3 wa s obtained the volume of N2 present in the container is a 13 33ml b 36 67ml c 26 67ml

Physical Chemistry
GeneralWhich of the following samples contains the largest number of atoms C 1 g of N g A 1 g of Ni s B 1 g of Ca s Which of the following contains greatest number of oxygen atoms A 1 g of O B 1 g of 0 2 all have the same number of atoms D 1 g of B s

Physical Chemistry
GeneralC H OH CHCI 1 Dichloromethyl 2 Dichlorocarbene CCL 3 Trichloromethyl anion CCI 4 Formyl cation CHO NaOH Salicylaldehyde the electrophile involved in the above reaction cation CHCI

Physical Chemistry
General11 7 g of impure sodium chloride is treated with excess of AgNO3 solution 14 35 g of silver chloride M 143 5 g mol precipitate is formed What is the percentage purity of sodium chloride A B C 40 50 60 20

Physical Chemistry
GeneralIn ground state of Cu The no of shells occupied subshells filled orbitals and unpaired electrons respectively are P A 4 8 15 0 B 3 6 15 1 en fat anter zuchter HRT Thach Bik

Physical Chemistry
GeneralConsider the following statements a o nitrophenol forms intramolecular hydrogen bond b Boiling point of H S is greater than H O c Hydrogen bond is stronger than covalent bond The incorrect statement s is are a only a and c only a and b only b and c only

Physical Chemistry
GeneralConsider the following statements a Greater is the viscosity more slowly the liquid flows b Glass is an extremely viscous liquid c Sl unit of viscosity coefficient is N m The incorrect statement s is are b only c only b and c only a and c only

Physical Chemistry
General1g Mg was burnt in a closed vessel containing 2g oxygen Which of the following statement is correct 1 0 25g of Mg will be left unburnt 2 0 33gofoxygen will be left unreacted 3 2 5g of MgO will be formed mix will weigh 3g