General Questions and Answers

Physical Chemistry
General3 A mixture of 0 5 mole of CO and 0 5 mole of CO is taken in a vessel and allowed to effuse out through a pinhole into another vessel which has vacuum If a total of A mole has effused out in time t show that M A M 1 A 36 where M and M are mean molar masses of the mixture that has effused out and the mixture still remaining in vessel respectively

Physical Chemistry
GeneralIn victor Mayer s method 0 2 g of a volatile compound on volatilisation gave 56 mL of vapour at STP Its molecular weight is 1 40 2 60 3 80 4 120

Physical Chemistry
Generalof its low solubility and K Cr O7 is separated What weight of chromate ore having 20 purity should be used to produce 1Kg potassi dichromate crystals A 3 81kg C 2 80kg B 1kg D 7 62kg The composition of air which is passed in Isten is 80 N and 30 For th 294

Physical Chemistry
General108 510 mg of a liquid on vapourisation in Victor Mayer s apparatus displaces 67 2 cc of dry air at NTP The molecular weight of liquid is 1 130 2 17 3 1700 4 170

Physical Chemistry
General15 A vessel of volume 8 0 x 103 m 3 contains ideal gas at 300 K and 200 kPa The gas is allowed to leak till the pressure falls to 125 kPa Calculate the mole of gas leaked out if temperature remains constant Assume ideal nature

Physical Chemistry
GeneralAcetic acid dissociates 1 3 What will be the pH of N 10 solution of the acid A 2 066 B 1 300 C 2 086 D 2 886

Physical Chemistry
General5g of pure MgO obtained by reaction of metallic magnesium with oxygen contains 3g of Mg Again 8 5 g of pure MgO obtained by heating MgCO contains 5 1g of Mg Show that these results are in accordance with the law of constant proportions

Physical Chemistry
Generala Derive a relationship between molality molarity of a solution in which w g of solute of molar mass M g mol is dissolved in W g solvent density of resulting solution d g ml 1 4 g mL b Calculate molality of 1 2 M H SO4 solution If its p


Physical Chemistry
GeneralCalculate wt of urea which must be dissolved in 400 g of water so final solutions has V P 2 less than V P of pure water Let V P be V of water P P 02 V
Physical Chemistry
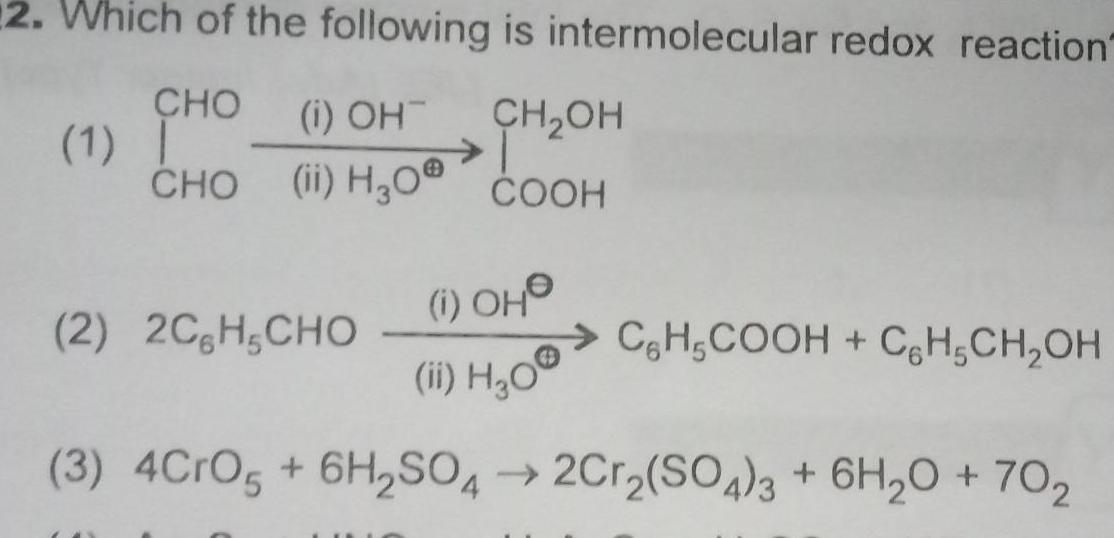
General2 Which of the following is intermolecular redox reaction CHO i OH CH OH CHO ii HgO COOH 1 1 OH ii H O 3 4CrO5 6H SO4 2Cr SO4 3 6H O 702 2 2C H CHO CH5COOH CH CH OH

Physical Chemistry
General4 9 47 m 7100 gm of a mixture of CaCO3 and Na CO was ignited strongly which forms 11 2 L of CO at STP Then the mass ratio of Na CO3 and Caco in the mixture will be 1 1 4 3 2 1 2 4 1 4 1 1 llowing has maximum number o

Physical Chemistry
General29 28 30 31 32 1 33 B 1 litre Which of the following has the highest mass A 1 g atom of C B mole of CH4 10 2 D 3 01 x 1016 condensed to water occupies an approximate volume of C 1 ml D 18 ml C 10 ml of water A person adds 1 71 gram of sugar C12H22011 in order to sweeten his tea The number of carbon atoms added D 3 011 1023 atoms of oxygen are mol mass of sugar 342 A 3 6 1022 B 7 2 x 1021 C 0 05 D 6 6 10 2 500 ml of a gaseous hydrocarbon when burnt in excess of O gave 2 5 It of CO and 3 0 It of water vapours under same conditions Molecular formula of the hydrocarbon is A C4H8 B C4H10 C C5H10 D C5H12 On analysis a certain compound was found to contain iodine and oxygen in the ratio of 254 80 The formula of the compound is At mass I 127 O 16 A 10 B 1 0 C 1502 D 1 05 A compound contains 38 8 C 16 0 H and 45 2 N The empirical formula of the compound would be A CH3NH C C H5CN D CH2 NH 2 B CH3CN A giant molecule contains 0 25 of a metal whose atomic weight is 59 Its molecule contains one atom of that metal Its minimum molecular weight is A 5900 B 23600 C 11800 D 100 59 0 4 Element A reacts with oxygen to form a compound A O3 If 0 359 gram of A react to give 0 559 gram of the tomic weight of A will be D 47 9

Physical Chemistry
General9 A mixture of N and H is caused to react in a closed container to form NH3 The reaction ceases before any of the reactant has been totally consumed At this stage 4 moles each of N H and NH3 are present Then the weight of N and H present originally were respectively 1 224 g and 16 g 3 168 g and 16 g 2 168 g and 20 g 4 224 g and 20 g

Physical Chemistry
GeneralOxidation of succinate ion produces ethylene and carbon dioxide gases On passing 0 2 Faraday electricity through on aqueous solution of potassium succinate the total volume of gases at both cathode and anode at STP 1 atm and 273 K is JEE MAINS ONLINE 2016 1 8 96 L 2 2 24 L 3 4 48 L 4 6 72 L

Physical Chemistry
GeneralKTRA CREDIT It was found that 16 g SO were formed from the reaction of iron IV sulfide and

Physical Chemistry
GeneralCaCO3 decomposes to give CaO and CO if the masses of CaO and CO produced are 5 6 g and 4 4 g respectively by heating 12 g of an impure CaCO3 sample then the impurity of the sample will be 1 33 33 2 16 67 3 83 33

Physical Chemistry
General1 2 g mixture of two divalent metals A at wt 30 and B at wt 15 on reacting with dilute HCI of dide KEY o tosolution gives 2 24 L H gas at NTP then 2 composition of A in g 1 1 2 0 5 La 3 1 5 08 4 1 2 22 2 8

Physical Chemistry
GeneralA mixture of potassium chlorate oxalic acid and sulphuric acid is heated During the reaction which element undergoes maximum change in the oxidation number a S c Cl b H d C Prelims 2012

Physical Chemistry
GeneralYellow light emitted from a sodium lamp has a wavelength A of 580 nm Calculate the frequency v wave number and energy of yellow light photon

Physical Chemistry
General102 NaOH then nature of resulting solution and 0 6 N CAN normality of excess of reactant left is D 0501 M 10 1 Acidic N 5 N blom 2 Basic M N 5 mi of N

Physical Chemistry
Generalc 500 500 A sample of an alloy of silver weighing 0 50 g and containing 90 silver was dissolved in conc HNO3 and silver was analysed by Volhard method A volume of 25 ml of a KCNS solution was required for complete precipitation The normality of KCNS solution is Ag 108 a 4 167 c 3 136 b 0 167 d 0 125

Physical Chemistry
General19 For the reaction 2NO g 2NO g O g K 1 8x10 6 at 185 C The value of K for the reaction NO g NO g O g at the same temperature is S a 1 34 10 b 1 8 10 6 09x 10 3 d 1 8 106

Physical Chemistry
GeneralThe most stable complex among the following is 2 Co NH3 2 4 Mn NH3 2 1 Fe NH3 612 3 Ni NH3

Physical Chemistry
GeneralWhen an ideal gas undergoes unrestricted expansic no cooling takes place because the molecules a Exert no attractive forces on each other b Do work equal to loss of KE c Collide without loss of energy d Are above the inversion temperature

Physical Chemistry
General5 Tyndall effect is more pronounced in a hydrophilic sols c lyophilic sols b hydrophobic sols d Both a and c

Physical Chemistry
GeneralAn equilibrium mixture at 700 K of 0 50 M N 3 00 M H and 2 00 M NH is present in a container Now if this equilibrium is disturbed by adding N so that its concentration becomes 1 50 M just after addition then which of the following graphs represents the above situation more appropriately Initial og Now oq Initial og Now oq A 2 0 1 0 C 3 0 2 0 1 0 Time Initial eq New eq Time NH NH B D 1 conc Conc M 2 0 1 0 3 0 2 0 1 0 Time Intial og New oq NH H N NH H N Timo 050 M N 3 00 MH 2 00 M NH fer

Physical Chemistry
GeneralWhich of the following changes with increase in temperature AIEEE 2002 a Molality b Weight fraction of solute c Fraction of solute present in water d Mole fraction

Physical Chemistry
Generaln moles of an ideal triatomic linear gas undergoes a process in which the temperature changes with volum as T k V where k is a constant Choose incorrect alternative 5 A At normal temperature C R BAt any temperature C C R C At normal temperature molar heat capacity C 3R DAt any temperature molar heat capacity C 3R

Physical Chemistry
Generalis due to adsorption of a H b S c OH 72 At CMC the surfactant molecules a decompose c associate 3 d 0 74 d b become completely soluble d dissociate and 5 itsad Arsenic sulphide in mering sol The reagent with least is negative

Physical Chemistry
GeneralGiven X Na HASO Y NaBrO ZHCl NaBr H AsO NaCl The values of X Y and Z in the above redox reaction are respectively 2 3 1 6 1 2 1 3 3 2 1 2 JEE Main o 4 3 1 4
Physical Chemistry
General21 1 4 g of an organic compound was digested according to Kjeldahl s method and the ammonia evolved was absorbed in 60 mL of M 10 H SO4 solution The excess sulphuric acid required 20 mL of M 10 NaOH solution for neutralization The percentage of nitrogen in the compound is 1 3 2 5 3 10 4 24

Physical Chemistry
General6 02 x 1020 molecules of a substance weigh 44 mg What is the molar mass of the substance

Physical Chemistry
General2 molecules ions 1 XeF4 4 SiF4 2 BF AIEEE 2006 The decreasing values of bond angles from NH3 1069 to SbH3 919 down group 15 of the periodic table is due are all the bonds not equal 3 SF4 to 1 decreasing lp bp repulsion 3 increasing bp bp repulsion 2 increasing electronegativity 4 increasing p orbital character in sp In which of the following ionization process the bond order has increased and the magnetic behaviour has changed AIEEE 2007

Physical Chemistry
GeneralIf 100 mL of 0 100 M Na SO is added to 200 mL of 0 150 M NaCl what is the concentration of Na ions in the final solution Assume that the volume are additive A 0 133 M B 0 167 M C 0 250 M D 0 350 M illilitan of 0 300 M phosphoris

Physical Chemistry
GeneralNo of oxalic acid molecules in 100 mL of 0 02 N oxalic acid are a 6 023 1020 c 6 023x1022 b 6 023x1021 d 6 023 x 1023

Physical Chemistry
GeneralIf uncertainty of position of electron is zero the uncertainty in its momentum would be a Zero b h 2n c h 4 d x 8

Physical Chemistry
GeneralA beam of specific kind of particles of velocity 2 1 x 107 m s is scattered by a gold nuclei Find out specific charge charge mass of this particle if the distance of approach is 2 5 10 4 m a 4 84 107 C kg b 4 84 x 10 7 C kg c 2 42 x 107 C kg d 3x10 12 C

Physical Chemistry
GeneralThe composition What percentage III of a sample of wustite is Fe 0 9301 of iron is present in the form of IIT 199

Physical Chemistry
GeneralNumber of atoms in 558 5 g Fe at wt 55 85 is AIEEE 2002 a Twice that in 60 g carbon b 6 023x1022 c Half in 8 g He 5022x 1023

Physical Chemistry
General4 Select the incorrect 1 Extensive properties are additive in nature 2 Intensive properties are mass independent 3 Vapour pressure is an extensive property 4 Pressure is an intensive property

Physical Chemistry
GeneralThe density of 3M solution of sodium thiosulphate Na S O is 1 58 g ml Calculate amount of Na S O in w w i mole fraction of Na S O iii molality of Na and S O2 ions Calculat

Physical Chemistry
General51 Which of the following is not primary standard a Na CO3 10H 0 b Oxalic acid 10H 0 d NaOH 52 c Na B407 The mole

Physical Chemistry
General100 ml of 3 mol H SO reacts with 100 ml of 3 mol NaOH Enthalpy of neutralisation of reaction will be 1 57 1 kJ mol 3 0 3 x 57 1 kJ 2 2 x 57 1 kJ 4 3 57 1 kJ

Physical Chemistry
GeneralWhich has minimum number of atoms of oxygen Kame X10 ml H 0 e 2 0 1 mol of V 05 s Begr 3 12 gm O g 4 12 04 x 1022 molecules of CO I male 1

Physical Chemistry
General16 In aqueous solution the ionization constants for carbonic acid are K 4 2 107 and K 4 8 10 1 Select the correct statement for a saturated 0 034 M solution of the carbonic acid 2 1 The concentration of H is double that of CO 2 The concentration of CO2 is 0 034 M 2 3 The concentration of CO3 is greater than that of HCO3 4 The concentrations of H and HCO3 are approximately equal AIEEE 2010 I will Motions start precipitating

Physical Chemistry
GeneralFrom the following bond energies H H bond energy 431 37 kJ mol C C bond energy 606 10 kJ mol C C bond energy 336 49 kJ mol C H bond energy 410 50 kJ mol Enthalpy for the reaction H H H H C C H HH C C H H H will be 1 553 0 kJ mol 1 OH H 2 1523 6 kJ mo

Physical Chemistry
General526 3 mL of 0 5 m HCl is shaken with 0 5 g of activated charcoal and filtered The concentration of the filtrate is reduced to 0 4 m The amount of adsorption x m is

Physical Chemistry
General5 Chloride samples are prepared for analysis by using NaCl KCl and NH4Cl separately or as mixture What minimum volume of 5 by weight AgNO3 solution sp gr 1 04g mL must be added to a sample of 0 3g in order to ensure complete precipitation of chloride in every possible case

Physical Chemistry
Generalc 90 a 0470 0 10 g of a sample containing CuCO and some inert impurity was dissolved in dilute sulphuric acid and volume made up to 50 mL This solution was added into 50 mL of 0 04 M KI solution where copper precipitates as Cul and I is oxidized into I3 A 10 mL portion of this solution is taken for analysis filtered and made up free I3 and then treated with excess of acidic permanganate solution Liberated iodine required 20 mL of 2 5 mM sodium thiosulphate solution to reach the end point Determine mass percentage of CuCO3 in the original sample a 7 41 c 61 75 b 74 1 d None of these