General Questions and Answers

Physical Chemistry
GeneralA and 1 55 g of B molar weight is 160 g mol are placed in a 1 00 L reaction vessel and sealed the mixture reacts and the following equilibrium is established 2A 9 B g 2C 9 At 25 C the equilibrium pressure of C is 0 264 atm What is K

Physical Chemistry
GeneralIn two vessels of 1 L each at the same temperature 1 g of DCE 2007 H and 1 g of CH4 are taken for these a Vs values will be same rms b Kinetic energy per mol will be same c Total kinetic energy will same d Pressure will be same

Physical Chemistry
GeneralFor a reaction of order n what is the relationship between t3 4 and where t is the time required for concentration C to become 1 4 C and Care the values of the reactant concentration at the start and after time t respectively 1 13 4 1 2 2 1 1 3 3 4 1 2 2 1 11 2 3 4 2120 1 1 4 3 4 1 2 2 1 1

Physical Chemistry
General3 C Energy of the electron in Hydrogen atom is given by a En c En 131 38 2 n 1313 3 2 n kJ mol b E AMU Engg 2002 131 33 kJ mol kJ mol d E n 313 13 2 n kJ mol P31

Physical Chemistry
General10 mole of ideal gas expand isothermally and reversibly from a pressure of 10 atm to 1 atm at 300 K What is the largest mass which can lifted through a height of 100 meter a 31842 kg b 58 55 kg c 342 58 kg d None of these 0

Physical Chemistry
General2 A device for sustained drug delivery is of the shape of a thin slab Both the top and bottom surface with an area of 1 5mm each are covered by polymeric membranes to control diffusion The core is a drug reservoir with very high drug concentration at 20 mg ml The device has a constant drug release rate of 2 g day a What is the permeability of the membrane for the drug State all assumptions in your calculation

Physical Chemistry
GeneralTwo drugs have a selectivity factor a 1 08 and capacity factors k 5 0 and k 5 4 If the plate height is 0 20 mm how long must the column be for a resolution of 1 5 A 22 3 m B 87 4 m 34 5 m D 17 3 m

Physical Chemistry
Generala 8 Write a detailed stepwise mechanism to explain ONLY ONE of the following reactions 5 points DO NOT DO MORE THAN ONE acetic acid OH HO isoamyl alcohol H O heat bu isoamyl acetate 9
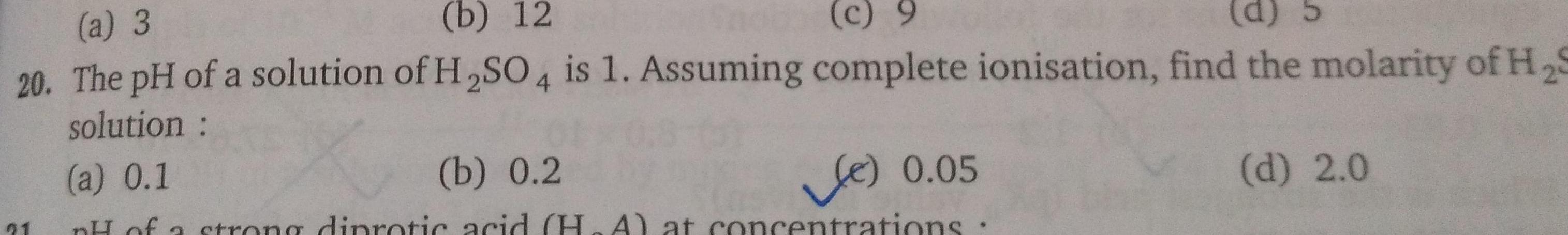
Physical Chemistry
Generala 3 b 12 c 9 d 20 The pH of a solution of H SO4 is 1 Assuming complete ionisation find the molarity of H S solution a 0 1 b 0 2 Le 0 05 pH of a strong diprotic acid H A at concentrations 21 d 2 0

Physical Chemistry
GeneralAt constant volume for a fixed number of a moles of a gas the pressure of the gas increases with the rise in temperature due to 1 Increase in average molecular speed 2 Increase in rate of collisions amongst 3 Increase in molecular attraction 4 Increase in mean free path

Physical Chemistry
GeneralA chemist wants to prepare phosgene COCI by the following reaction CO g Cl g COCI g He places 2 20 g of chlorine Cl and an equal molar amount of carbon monoxide CO into a 10 00 L reaction vessel at 395 C After the reaction comes to equilibrium he adds another 2 20 g of chlorine to the vessel in order to push the reaction to the right to g more product What is the partial pressure of phosgene when the reaction again comes to equilibrium K 1 23 x 10 Partial pressure atm

Physical Chemistry
General6 In Partition coefficient experiment aqueous layer comes below n Butanol layer because O O O O a The density of water is less than n Butanol b Acetic acid is more soluble in n Butanol layer c The density of water is more than n Butanol d Acetic acid is more soluble in aqueous layer

Physical Chemistry
GeneralAs the volume of HCl and NaOH is increased containing 25 mL 0 5M acetic Acid Acetate Buffer describe the effect on the pH Did the buffer stop working at some point If so at what volume of acid and base did the buffer fail Why

Physical Chemistry
GeneralMark O if the statement is true X if false For the false statements correct them a Carbon coked catalysts can be reused after treatment with hot steam b For a certain catalytic reaction where A is converted to either B and C if the differential selectivity with respect to product B is 0 5 the production rate of C is twice faster than that of A c Attrition is a type of irreversible deactivation of catalysts d The most appropriate type of reactor in the conversion of methane to hydrogen by reacting with gaseous water is single pass fixed bed reactor

Physical Chemistry
GeneralA 0 846 M KMnO4 solution was used to determine the hydrogen peroxide H O2 MM 34 016 content in a 25 48 mL of peroxide solution It took 13 28 mL of the KMnO4 solution to reach the endpoint What is the percentage w v of H O2 in the sample The reaction is 2 5 H O 2 MnO4 6 H 5 O 2 Mn 8 H O

Physical Chemistry
GeneralRefer to the given graph plotted for three different fabric pieces of equal size P Q and R on the basis of their water absorbing capacity and select the correct statement Amount of water absorbed 0 Fabric A P is made up of fibres obtained from Gossypium plant whereas R is made up of fibres obtained from an insect BQ is made up of protein fibres obtained from an insect whereas R is made up of cellulose fibres obtained from flower of a plant C R is made up of fibres obtained from plant stem by the process of retting whereas P is made up of fibres obtained from plant seed by the process of ginning

Physical Chemistry
GeneralA solution containing 0 100 mol of hydrochloric acid is added to 8 43 g of magnesium carbonate MgCO3 s 2HCl aq MgCl aq CO g H O l a What is the total volume at r t p of carbon dioxide formed M MgCO3 84 3 A 2 40 dm B 1 20 dm C 2400 dm D 1200 dm Molar volume of a gas at r t p 24 0 dm mol

Physical Chemistry
GeneralHow many of the following compounds are more acidic than water OH a OH 0 NO c i methanol NO g ethanol h isopropyl alcohol OH d 0 NO OH 0 min sec e OH f OCH OO CH

Physical Chemistry
GeneralBased on the equation CH4 g 202 g CO2 g 2H O g what is the change in internal energy if 4 5 grams od CH4 was oxidized at 25 C Select one O a 190 15 kJ b 195 68 kJ C 200 63 kJ d 199 24 kJ AH 802 kJ

Physical Chemistry
GeneralA 0 3146 g sample of a mixture of NaCl s and KBr s was dissolved in water The resulting solution required 46 00 ml of 0 08765 M AgNO aq to precipitate the CI aq and Br aq as AgCl s and AgBr s Calculate the mass percentage of NaCl s in the mixture

Physical Chemistry
GeneralConsider the following reaction 3 A 7 B 4D 2E If 17 moles of A 42 moles of B and 33 moles of D are present Calculate moles of E produced at 50 completion of reaction O 5 67 O 8 5 O 11 33 O 9 7

Physical Chemistry
General13 9 g of FeSO4 xH O crystals were dissolved in a dilute sulphuric acid and volume made up to 1000 ml 25 ml portion of this solution required 50 ml and 0 005 M KMnO4 solution to reach the end point The value of x to the nearest integer is Fe 56 S 32 0 16 g mol Answer Enter your answer here Back Space

Physical Chemistry
GeneralFT IR cm 1 740 H NMR 84 35 1H quartet 4 25 2H quartet 1 8 3H doublet 1 3 3H triplet 1 C NMR DEPT CH CH3 CH 8 62 1 40 1 22 1 15 Mass spectrum m e 180 182 M 152 154 107 109 determine the structure of C5H902Br

Physical Chemistry
General36 The initial concentration of the reactant in a first order reaction is a It takes time t for the completion of n th fraction of the reaction Will it take time 2t for the completion of the same fraction if the initial concentration of the reactant is made twice

Physical Chemistry
GeneralCOOH OH CH CO OH Salicylic acid Common name of the compound formed in by above reaction will be O Aspirin Oil of winter green Salol

Physical Chemistry
General1 i ii Features a Thick waxy covering b Large fleshy stems c Spikes d Shallow roots that spread out a long way Define endangered 3 How the feature helps the cactus survive in the desert

Physical Chemistry
GeneralIf a hydrate of Ba CIO4 2 is heated to 250 C all the water of hydration is lost On heating a 1 687 g sample of the hydrate 1 453 g of Ba CIO4 2 remains How many molecules of water occur per formula unit of Ba CIO4 2

Physical Chemistry
GeneralThe resistance of a 0 1 M solution of a weak acid HA in a cell was found to be 600 ohm What will be its pH at the same concentration Given cell constant 0 936 cm 1 M NaCl 126 S cm mol A M HCI 426 S cm mol A M NaA 90 S cm mol A 3 log 4 C 5 log 4 B 2 log 4 D 6 log 4

Physical Chemistry
General3 Write chemical equations for the following reactions a Calcium hydroxide reacts with nitric acid b Acetic acid reacts with calcium hydroxide e Hydrochloric acid reacts with sodium hydroxide d Ammonium hydroxide reacts with sulphuric acid ca x z

Physical Chemistry
GeneralThe van t Hoff factors for mercurous chloride and mercuric chloride are respectively unity and greater than unity Which of the following statements is true about two chlorides 0000 Mercuric chloride is covalent Dissociation of HgCl2is incomplete while mercurous chloride is complete Mercurous chloride is sparingly soluble in water Both mercuric and mercurous chlorides are covalent

Physical Chemistry
Generalsec Which of the following alkene will not be formed on dehydration of 3 methylhexan 2 ol

Physical Chemistry
GeneralWBSU Year 2018 5 a How will the advancement of reaction S change for N2O4 g 2NO2 g when i 5 mol of Ar introduced keeping mixture at constant P ii 5 mol of Ar introduced keeping mixture at constant V 4

Physical Chemistry
GeneralRead the information about all the makers of the Indian Constitution given in the side columns here You don t need to memorise this information Just give examples from these to support the following statements 1 The Assembly had many members who were not with the Congress 2 The Assembly represented members from different social groups 3 Members of the Assembly believed in different ideologies

Physical Chemistry
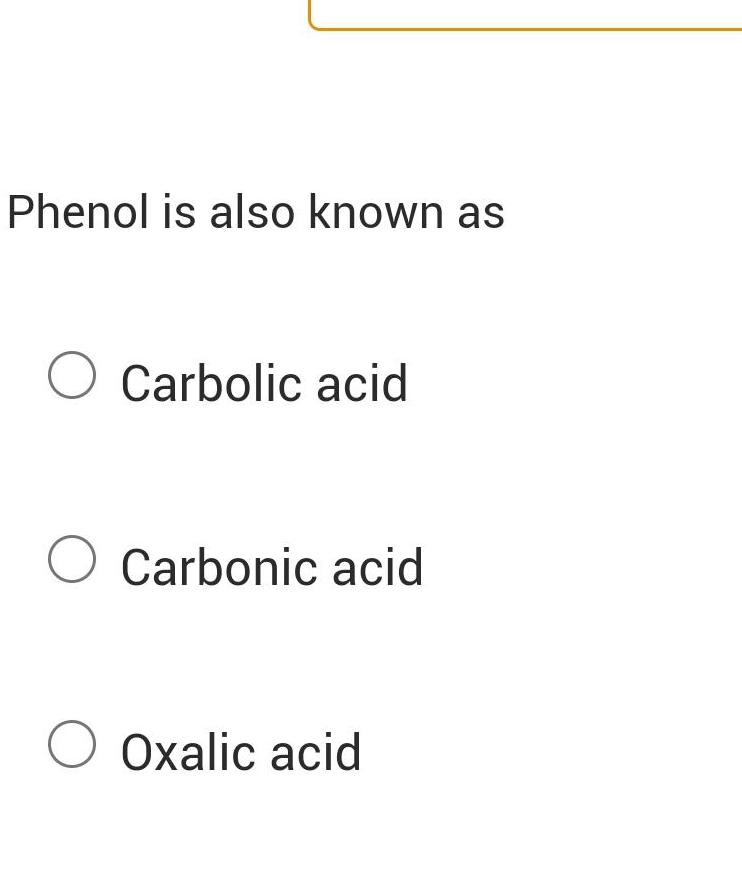
GeneralPhenol pKa 9 95 and sodium bicarbonate pKa 10 2 react to form an equilibrium with conjugate base of phenol and carbonic acid pKa 6 4 What is the Keq OA 1 58 x 10 4 OB 0 000282 OC 3 80 OD 2 51 x 10 4 OE 1 58 x 10 4 OF 1 58

Physical Chemistry
GeneralThe correct statement about SO3 and ClOzis i Both are isoelectronic iii Both are not isoelectronic O O ii ii O i ii i iv iii iv ii Both are isostructural iv Both are not isostructural

Physical Chemistry
GeneralYou are a biotechnologist designing a new elimination reaction Based on the retrosynthetic analysis you completed there s a possibility that you will have E and E competing mechanisms in your reaction What is the best way to set up the laboratory experiment so that you can differentiate between E and E mechanisms Specify how would you experimentally determine the mechanism of your new reaction 5 marks

Physical Chemistry
GeneralIf a 10 gm sample of dry ice is kept in a bottle of 2 L capacity at 27 C and at a time pressure is found to be 760 mm of Hg then find the approximate weight of dry ice left Only One Correct Answer A B 6 4 gm 3 2 gm 2

Physical Chemistry
GeneralIve done practice problems where these questions were worded differently and between 2 answers for them If someone can explain what the answer is so I can understand it for my next exam 14 Which of the following is the B D gulopyranose HO A B C D E HO A OH 2 1 0 OH OH 1 2 HO H B OH OH OH HO HO C OH OH OH HO H HO D OH OH HO H E OH 15 What is the overall net charge on the pentapeptide CSKKG at pH 8 2 OH OH

Physical Chemistry
GeneralCan we say in adiabatic process if change i n heat is zero then change in temp will ulti mately be zero as zeroth law says that energy flows in for m of heat when there is a temp differnce h ence if there is no change in heat there will be no temp difference

Physical Chemistry
Generalgiven setup burner test tube stand the chemicals used and their pro test Tube jas jar 75 through of water LABORATORY PREPERATION OF OXYGEN A Potassium chlorate and Manganese dioxide 5 1 B Potassium chlorate and Nickel S 1 C Potassium chlorate and Manganese dioxide Is inickel 1 5

Physical Chemistry
GeneralState whether each of the following statements is TRUE or FALSE and explain your answer a A MALDI TOF mass spectrometer with the upper mass limit of m z 1500 is not able to analyze a protein of mass 15 000 Da 4 marks b ESI is preferred over MALDI in some chemical analyses 4 marks

Physical Chemistry
GeneralUsing your knowledge of the hard soft acid base HSAB theory predict with reasoning whether you would expect the following reactions to occur i Fe acac 3 Fe Fe acac 3 Fe acac is the acetylacetonate anion ii Ni CO 4 2 e Ni CO 4 iii Hg OH 2 Mn SH 2 Hg SH 2 Mn OH 2 iv AgCl 2 CN Ag CN 2 2 CI

Physical Chemistry
GeneralYou are examining a diatom sample using a BH2 polarizing light microscope with the 0 40 numerical aperture 20X magnification objective lens in air but you re not able to resolve the fine details of the sample with these settings Describe three different specific methods that you can use to improve the resolution of detail of this sample Explain in detail how each of these methods works to improve resolution of detail You can assume that the microscope has already been set up for K hler illumination

Physical Chemistry
GeneralFor reversible Galvanic cell thermodynamic parameters are given as AG Tp AH TAS 4 AG Tp nFEcell nF de cell dT AS Wusefull AG T P For given cell Pt H HCI AgCl Ag variation of e m f of the cell with temperature is given as Ecell in volt 0 4 2 10 T T temperature in Kelvin Use 1F 96500 C P For given cell magnitude of entropy change occured for production of one mole of Ag in J K at 27 C will be

Physical Chemistry
General16 Given the following reactions 1 CH O CH O H 2 CH O N 3H 3 N 3H 2NH the AH for N H H 2NH is A 18 0 B 75 0 N H CHO C 148 AH 65 0 kJ AH 37 0 kJ AH 46 0 kJ kJ D 192 E 18 0

Physical Chemistry
GeneralStatement 1 Every 100 ml of oxygenated blood can deliver 5 ml of O to the tissue under normal physiological conditions Statement II Every 100 ml of deoxygenated blood deliver approximately 4 ml of CO to alveoli A Statement I is correct and Statement II is wrong B Statement I is wrong and Statement II is

Physical Chemistry
GeneralCH CO H An analytical chemist has determined by measurements that there are 8 483 moles of carbon in a sample of acetic acid How many moles of hydrogen are i sample Round your answer to 4 significant digits 0 9

Physical Chemistry
GeneralYou are asked to dissolve 38 45 g of Na2HPO4 in 450 0 mL of distilled water Please determine the answers to the following four concepts and select the most appropriate answer for each concept from the list below 4 answers should be selected a m v b Concentration in mol L c Reaction capacity d Normality N

Physical Chemistry
GeneralChem Section B This section contains NUMERICAL VALUE TYPE questions Enter the correct numerical value in Read More A sample of hydrazine N H4 was dissolved in 0 1 L of water 5 ml portion of this solution was reacted with excess of FeCl3 solution and covered to complete the reaction Ferrous ions formed were estimated and it M requires 10 ml of KMnO4 solution 50 Given 4Fe N H4 N 4Fe2 4H KMnO4 5Fe 8H Mn 5Fe 4H O The amount of hydrazine in g present in 1 liter of solution is x Then the value of x is