General Questions and Answers

Physical Chemistry
GeneralHigh penetration effect of ns electrons as compared to np electrons is responsible for Question Type Multiple Correct Type 1 2 Higher IE2 of Na as compared to Mg 3 Higher IE of Mg as compared to Al 4 Higher IE of P as compared to S Higher IE2 of B as compared to C

Physical Chemistry
GeneralWhich of the following statements is incorrect 8 for the reaction Excess of KI reacts with CuSO4 solution and NaS O3 solution is added to it 1 Evolved I is reduced 2 Cul is formed 3 Na S O3 is oxidised 4 Cu Io is formed

Physical Chemistry
GeneralWhat volume of 4 molar NaOH can be prepared from 5 molar 2 litre solution of NaOH 250 litre 2500 ml 5000 ml

Physical Chemistry
General28 If average molecular wt of air is 29 then assuming N2 and O gases are there which options 2 are correct regarding composition of air iii 72 41 by mass of N 2 only ii is correct b d both i and ii are correct 2 i 75 by mass of N ii 75 by moles N a only i is correct c both ii and iii are correct

Physical Chemistry
Generalatomic volume that is occupied by nucleus is kx 10 The value of k is Ethylene oxide C H O is manufactured by burning of ethylene in air 2C H O 2C H O 60 g of ethylene oxide is obtained from 42 g of ethyline Calculate the precentage yield

Physical Chemistry
General17 Calculate the weight of CO having the same number of oxygen atoms as are present in 2 of CO 28 g 18 What is the molecular mass of substance each molecule of which contains 4 atoms of can parts of Mass

Physical Chemistry
GeneralBy a sample of ground state atomic hydrogen UV light of energy spectrum Orrect Answer 5 00 13 6 x 48 eV 49 is absorbed How many different wavelengths will be observed in Balmer region of hydrogen quanta

Physical Chemistry
GeneralFor a consecutive reaction R A k B R K If initial concentration of R is 100 M and k k 1 0 15 what is the value of tmax Given k 4 0x10 2 min 1 R3 80 55 min 55 80 min

Physical Chemistry
GeneralYou want to determine the accuracy of your analytical method You purchase a certified sample that contains 17 3 ppm of your analyte You measure the concentration in this sample three times and obtain concentration values of 15 4 15 2 and 15 6 ppm What is the absolute accuracy of your analysis

Physical Chemistry
Generali PCI g PCI g Cl g and ii SO CI g So g Cl g are simultaneously in equilibrium in an equilibrium box at constant volume A few moles of PCI C are later introduced into the vessel After som time the new equilibrium concentration of 1 SO Cl will remains unchanged 2 Cl will be greater 3 SO Cl will become less A SO CL will become greater

Physical Chemistry
GeneralPbo PbS T t 16 Galena an ore is partially oxidized by passing air through it at high temperature After some time the passage of air is stopped but the heating is continued in a closed furnace such that the 2018 contents undergo self reduction The weight in kg of Pb produced per kg of O2 consumed is Atomic weights in g mol 1 O 16 S 32 Pb 207

Physical Chemistry
GeneralAn ideal gas is expanded from p V T to P2 V T under different conditions The correct statement s among the following is are Question Type Multiple Correct Type 1 2 3 4 The work done on the gas is maximum when it is compressed irreversibly from P2 V to P V against constant pressure p The work done by the gas is less when it is expanded reversibly from V to V under adiabatic conditions as compared to that when expanded reversibly from V to V2 under isothermal conditions The change in internal energy of the gas i zero if it is expanded reversibly with T T and ii positive if it is expanded reversibly under adiabatic conditions with T T2 If the expansion is carried out freely it is simultaneously both isothermal as well as adiabatic

Physical Chemistry
GeneralChemistry Question No 54 Question Palette The minimum volume of 20 volume H O solution that decolourises 200 mL of 2N KMnO solution in acidic medium is

Physical Chemistry
GeneralWhen 1 0 kg of anthracite coal is burned about 7300 kcal is evolved What amount of coal is required to heat 4 0 kg of water from room temperature 20 C to the boiling point at 1 atm pressure assuming that all the heat is available 320 g 32 g 44 g 440 g

Physical Chemistry
Generalb Air 79 mol nitrogen and 21 mol oxygen is passed over a catalyst at high temperature Oxygen completely reacts with nitrogen as shown below 0 5 N2 g 0 5 02 g NO g 0 5 N2 g O2 g NO2 g The molar ratio of NO to NO2 in the product stream is 2 1 What is the fractional conversion of nitrogen C One mole of methane undergoes complete combustion in a stoichiometric amount of air The reaction proceeds as CH4 202 CO2 2H2O Both the reactants and products are in gas phase AH 298 730 kJ mole of methane If the average specific heat of all the gases vapor is the maximum temperature rise of the exhaust gases in OC would be approximately equal to Assume the reference temperature 298 K 40 J mol K 1

Physical Chemistry
GeneralProblem No 30 Find the spectral terms of np electrons and compare the results obtained with t spectral terms obtained using Breit s scheme Write the spectral terms in the order the increasing energy values

Physical Chemistry
GeneralThe potential energy W of a system of two atoms A B varies as a function of their distance of separation r as follows The bond dissociation energy of A B bond DA B is given by A 1 DA B 2 DA BE 3 DA B 4 DA B A A m n n 1 m n

Physical Chemistry
GeneralConsider the four statements below Which of these best describes a theory A statement of a possible explanation for observed phenomena D A procedure for making controlled observations An explanation that is trusted because repeated tests have confirmed its validity d A summary of observed regularity in nature often as a mathematical equation

Physical Chemistry
GeneralAn element crystallizes into a structure which may be described by a cubic type of unit cell having one atom on each corner of the cube and two atoms on one of its body diagonals If the volume of this unit cel a is 24x10 24 cm and density of element is 7 2 g cm calculate the number of atoms present in 200 g o element

Physical Chemistry
GeneralA polyvalent metal weighing 0 1 g and atomic weight 51 reacted with dil H SO4 to evolve 43 9 ml of H2 at STP This solution containing metal in lower oxidation state was found to require 58 8 ml of 0 1 N permanganate for complete oxidation Identify the higher oxidation state of the metal A 2 C 5 B 3 D 7

Physical Chemistry
Generala 46 g b 20 g 7 The total no of neutrons present in 54 mL H O I are c 24 N a 3 NA b 30 NA 8 Total no of electrons present in 48 g Mg2 are A d none of these d non of these

Physical Chemistry
General12 What is the percent by mass of titanium in rutile a mineral that contain Titanium and oxygen if structure can be described as a closet packed array of oxide ions with titanium in one half of the octahedral holes What is the oxidation number of titanium Ti 48

Physical Chemistry
GeneralO The interaction energy of London force is inversely proportional to sixth power of the distance between two interacting particles but their magnitude depends upon a charge of interacting particles b mass of interacting particles c polarisability of interacting particles d strength of permanent dipoles in the particles De dipol for CA Abween tele possessing permanent dipole Ends of lipoles posses partial charges Thertial charge is ha un lectronic charge a myri tec b equal to unit electronic charge

Physical Chemistry
GeneralThe polymerization of terephtalic acid and ethylene glycol is self catalyzed and carried out at 290 C The rate constant k is 2 65 105 dm5 s mol The monomers are added to the batch reactor in stoichiometric amounts a If we would like to produce PET with a number average molecular weight of 2 500 g mol within 7 hours what initial concentration of the monomers is needed

Physical Chemistry
GeneralHow many e are left over on the central atom of H SeS4 what is the formal charge fc on the central atom 2e 1 left over fc O 0 e 1 left over fc 0 0 e left over fc 1 O e 1 left over fc 2

Physical Chemistry
GeneralA 10 g mixture of K2CO3 MgCO3 on decomposition produces total 2 2 gm CO Mass of K2CO3 present in the mixture is Atomic mass C 12 O 16 Mg 24 K 39 A 42 B 5 8 C 58 D 22 A A

Physical Chemistry
GeneralF Na Mg 0 K Rb Cs 74 Be 7 Fr given below and answer the following questions PERIODIC TABLE OF THE ELEMENTS ww Ca Sc Sr Y Ba La Lu Hf 100 Ra Ac Lr Rf 41 LANTHAND ACTRONG V C Nb Mo Tc La Ce Pr 127 Db Sg Bh Hs Re Ac Th Pa Ru Rh R Ir AN 45 Ni Cu Zn Ga Ge As Pd Ag Cd Pt H Au Hg 1 What is this table called Who invented this table 2 Name the following a A metallic element 41 ww Nd Pm Sm Eu Gd Tb Dy Ho Np Pu Am Cm Bk b A non metallic element S Pb 3 Define atomic number of an element 4 What is the basis of arrangement of these elements in the table Ay Ds Rg Cn Uut FI Uup Lv Uus Uup Po 2 Br Kr c A noble gas At Rn CF Es Fm Md No Lr

Physical Chemistry
GeneralIf the radius of Mg2 ion Cs ion O2 ion S ion and Cl ion are 0 65 1 69 1 40 1 84 and 1 81 respectively Calculate the co ordination numbers of the cations in the crystals of MgS MgO and CsCl then of the hand

Physical Chemistry
General177 In the following colourless white sulphides is are CdS PbS HgS CuS FeS ZnS NiS Bi S 78 0 38 gm of a silver salt of a dibasic acid on ignition gave 0 27 gm of silver Molecular mass of acid is x x 10 gm then x value is 79 Find out number of alcohols that can give positive iodoform test CACHOU Ehi

Physical Chemistry
GeneralIn an Otto cycle air at 1bar and 290K is compressed isentropic ally until the pressure is 15bar The heat is added at constant volume until the pressure rises to 40bar Calculate the air standard efficiency and mean effective pressure for the cycle Take Cv 0 717 KJ Kg K and Runiv 8 314KJ Kg K

Physical Chemistry
GeneralWhich reaction does not represent auto redox of disproportionation 45 A Cl OH CI CIO3 H O B C D 2H O2 2Cu H O O2 Cu Cu NH4 2Cr2O7 N2 Cr2O3 4H O

Physical Chemistry
General8 A solution containing 10 g dm 3 of urea molecular mass 60 g mol is isotonic with a 5 solution of a non volatile solute The molecular mass of this non volatile solute is 2006 a 300 g mol c 200 g mol b 350 g mol 1 d 250 g mol

Physical Chemistry
GeneralConsider the following reactions where metal gas reacts with iodine gas aluminum 2 Al 312 Al216 If 17 0 grams of aluminum metal reacts with 225 0 grams of iodine gas to produce 82 5 grams of Al216 what is the percent yield

Physical Chemistry
General3 4 4 1 66 0 5 g of an organic substance was kjeldahlised and the ammonia released was neutralised by 100 ml 0 1 M HCI Percentage of nitrogen in the compound is 1 14 3 28 2 42 4 72
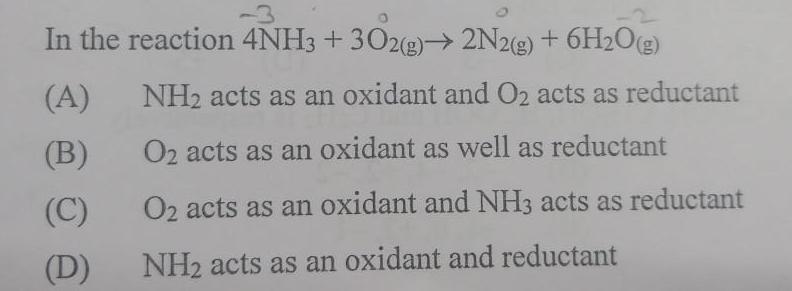
Physical Chemistry
GeneralIn the reaction 4NH3 302 g 2N2 g 6H O g A B C D NH acts as an oxidant and O2 acts as reductant O2 acts as an oxidant as well as reductant O2 acts as an oxidant and NH3 acts as reductant NH acts as an oxidant and reductant

Physical Chemistry
GeneralOne sample of atmospheric air is found to have 0 03 of carbon dioxide and another sample 0 04 This is evidence that 1 The law of constant composition is not always true 2 The law of multiple proportions is true 3 Air is a compound 4 Air is a mixture Number of moles of water in 1 litre of water with density 1g cc are 1 55 56 3 56 55 2 45 56 4 56 45 One nanometre is equal to 1 10 cm 3 10 7 cm 2 10 cm 4 10 cm 0

Physical Chemistry
GeneralChlorine is prepared in the laboratory by treating manganese dioxide MnO with aqueous hydrochloric acid according to the reaction 4HCl aq MnO s 2H O MnCI aq Cl g How many gram of HCI react with 5 0 g of manganese dioxide At wt of Mn 55 1 2 12 g 2 44 24 g 3 8 4 g 4 3 65 g

Physical Chemistry
General2 For the reaction N O5 g 2NO g 1 2 O g the value of rate of disappearance of N O is given as 6 25 10 3 mol L s 1 The rate of formation of 2 5 NO and O is given respectively as 2 AIPMT Prelims 2010 X 1 6 25 10 3 mol L s 1 6 25 x 10 3 mol L s 1 2 1 25 10 mol L s 1 3 125 10 mol L s 1 3 6 25 x 10 3 mol L s 1 3 125 x 10 3 mol L s 1 4 1 25 x 10 2 mol L s 1 6 25 10 mol L s 1 of activation is X

Physical Chemistry
GeneralThe chromate ion present in water sample is reduced to insoluble chromium hydroxide Cr OH 3 by dithionation in basic solution S 02 CrO2 2H O2SO3 Cr OH 3 OH 100 L of water requires 387 g of Na S O The molarity of CrO2 in waste water is 1 0 0448 3 0 0148 2 4 448 4 0 0224

Physical Chemistry
GeneralThe reaction between dinitrogen and dihydrogen is given as N2 3H2 2NH 3 What will be the mass of ammonia produced if 100 g of dinitrogen reacts with 500 g of dihydrogen 2833 a

Physical Chemistry
GeneralPay load is defined as the difference between the mass of displaced air and th mass of the balloon Calculate the pay load when a balloon of radius 10 m mas 100 kg is filled with helium at 1 66 bar at 27 C Density of air 1 2 kg m 3 an R 0 083 bar dm K 1 mol

Physical Chemistry
General4 Reduction of V O5 followed by addition of a strong base yields Na12V18042 24H O on crystallization if the addition of strong base causes no change in oxidation state of vanadium obtained after reduction the equivalent weight for reduction of V Os in this case will be if M denotes molecular weight of V 0s A M 2 B M S C M D Cannot determine

Physical Chemistry
General1 litre of a hydrocarbon weighs as much as one litre of CO The molecular formula of hydrocarbon is 1 C H 8 3 C H 4 Fou 2 C H 4 C H

Physical Chemistry
GeneralWhich of the following metal does not react with the solution of copper sulphate Zn Fe Ag Mg

Physical Chemistry
GeneralTwo moles of triatomic linear gas are taken through a reversible process starting from A as shown in figure The volume ratio V NAX 4 If the temperature at A is 73 C then total enthalpy change in both steps is X R joules X Report the value of Vol L 100 C B A Temperature K Correct answer 42 00

Physical Chemistry
GeneralWhich of the following statement about the composition of the vapour over an ideal 1 1 molar mixture of benzene and toluene is correct Assume that the temperature is constant at 25 C Given Vapour pressure data at 25 C benzene 12 8 kPa toluene 3 85 kPa 2016 a The vapour will contain a higher percentage of benzene b The vapour will contain a higher percentage of toluene The vapour will contain equal amounts of benezene and toluene d Not enough information is given to make a dication c

Physical Chemistry
GeneralA sample of certain mass of an ideal nonlinear polyatomic gas is expanded against constant pressure of 1 atm adiabatically from volume 2L pressure 6 atm and temperature 300 K to state where to state where its final volume is 8L Calculate entropy change in J K in the process Neglect vibrational degrees of freedom Take 1L atm 100J R 8 0 J mol K 0 08 Lt atm K mol log 2 0 3 log 3 0 48 log e 2 3 A 3 312 B 3 312 C 2 693 D 2 693

Physical Chemistry
GeneralWhen the gases sulphur dioxide and hydrogen sulphide m ix in the presence of water the product obtained are wate r and sulphur Here hydrogen sulphide is acting as A An oxidizing agent B Reducing agent

Physical Chemistry
GeneralThe second ionisation potential of Be is 17 98 eV If the electron in Bet is assumed to move in spherical orbit with a central field of effective nuclear charge Zeff consisting of the nucleus and other electrons If the extent of shielding by the K electrons of Li atom is same as calculated above the ionisation potential of Li will be 1 10 2 eV 3 5 746 eV 2 3 476 eV 4 3 2 eV

Physical Chemistry
General16 Which alum is a double salt made up of two salts 1 Salt of a SA WB Salt of a WA WB 3 Salt of a SA SB Salt of a WA WB 2 Salt of a SA SB Salt of a SA WB 4 Salt of a SA WB Salt of a WA WB