General Questions and Answers

Physical Chemistry
Generalhis section contains 5 questions The answer is a single digit integer ranging from 0 to 9 both inclusive Marking scheme 3 for correct answer 0 if not attempted and 0 in all other cases 2 K for CH COOH is 1 8 105 and K for NH4OH is 1 8x10 5 The pH of 0 01 M ammonium acetate will be Pure gas A was taken in a closed container at an initial pressure of 5atm the 33

Physical Chemistry
General4 A stock solution of 2 5 M H SO was found untouched at the bottom of a laboratory storage room Anna wants to know if the solution s pH level is perfect for her experiment Help Anna calculate for the A H B OH C pH and D pOH of this solution

Physical Chemistry
GeneralA balloon has a volume 400 ml at 0 C The balloon is distended to 4 5th of its maximum stretching capacity i Will burst if it is brought into a room at 40 C ii What is the maximum temperature above which it will burst

Physical Chemistry
General1 25 weights of oxygen which combine with a fixed weight of metal 0 64g in the two oxides 0 16 0 08 or 2 1 o is simple whole number in nature the law Multiple proportions is illustrated le 1 26 Copper gives two oxides On heating 1g of each in hydrogen we get 0 888 g e metal respectively Show that these results are in agreement with the Law of Multiple us fix 1g of copper as the fixed weight in the two oxides xide Weight of copper 0 888 g Weight of oxygen 1 0 888 0 112 g 0 112 g 888 g of copper combine with oxygen 1

Physical Chemistry
GeneralFor the electrons of oxygen atom which of the following statements is correct A Zeff for an electron in a 2s orbital is the same as Zeff for an electron in a 2p orbital B An electron in the 2s orbital has the same energy as an electron in the 2p orbital C Zeff for an electron is 1s orbital is the same as Zeff for an electron in a 2s orbital D The two electrons present in the 2s orbital have spin quantum numbers ms but of opposite sign

Physical Chemistry
GeneralWhich of the following statements is not true as per Dalton s atomic theory O Matter consists of divisible atoms O All atoms of a given element have identical properties O Atoms of different elements differ in mass O Chemical reactions involve reorganisation of

Physical Chemistry
Generalumber of times the resultan amplitude becomes maximum or minimum in one second 42 A tuning fork of unknown frequency produces 4 beats per second when sounded with another tuning fork of frequency 254 Hz It gives the same number of beats per second when unknown tuning fork loaded with wax The unknown frequency before loading with wax is 1 258 2 254 3 250 4 Can t be determined

Physical Chemistry
General4 An isochoric process If at STP velocity of sound in a gas y 1 5 600 m s the r m s velocity of the gas molecul at STP will be 1 400 m s 3 600 2 m s 2 600 m s 4 300 2 m s

Physical Chemistry
GeneralA and B are two different chemical species undergoing first order decomposition with rate constants K and kg which are in the ratio of 3 2 respectively If the initial concentration of A and B are in the ratio of A o B o 3 2 What would be the ratio of A B after three half lives of A Only one correct answer A 1 1 B 3 4 C 1 2
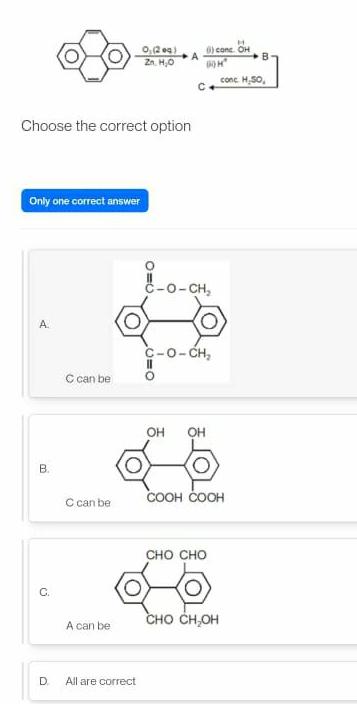
Physical Chemistry
GeneralChoose the correct option Only one correct answer A B C D C can be C can be A can be 0 209 Zn H O All are correct 0 0 II DOH C O CH C O CH OH OH conc OH CHO CHO COOH COOH CHO CH CH B conc H SO

Physical Chemistry
GeneralFrom the following facts 1 2X Y 2Y X ii 2W Y No reaction iii 2Z X 2X Z Which of the following is true Only one correct answer A Ew w Ey Y Ex x Ez z B Ew w Ey Y Ez Z Ex X C Ew w Ez 2 Ey Y Ex x D Ew w Ex X Ey Y Ez 2

Physical Chemistry
General22 Two samples A and B of a pure substance containing elements Y and Z are obtained from two different sources 5g of sample A contains 1 25 g of Z Sample B is made of 75 of Y by weight This is an illustration of which of the following laws forme 2 3 Law of constant proportion Law of multiple proportion X Law of mass conservation Avagadro s Law Y

Physical Chemistry
General3 54 N 4 0 0054 N A rope of length L and mass m hangs freely from the ceiling The velocity of transverse wave as a function of position x along the rope is proportional to 1 2 x 3 4 X

Physical Chemistry
GeneralQuestion Number 142 Question Id 8135611742 Question Type MCQ Display Question Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical The major product of the following reaction is CH CHBr 2 woje wassum Ja CH CHBr NaNH Red hot iron tube 873 K Options NaNH Jon Scdabar v exo 873 K CH CH CH CH C CH P P

Physical Chemistry
GeneralIn blast furnace during the extraction of Iron coke is used as fuel and burnt Now my Question is that is in Vertical Retort Process duri ng extraction of Zinc The CO and N2 are used as fuel and are burnt If yes how CO and N2 are burnt

Physical Chemistry
GeneralA graduated cylinder contains 15 4 mL of water What is the new water level in millilite after 30 6 g of silver metal is submerged in the water Express your answer using three significant figures AZO

Physical Chemistry
GeneralSelect all FALSE statements regarding 1 mol of C6H6 molecules It contains same number moles of i e 6 of carbon and hydrogen atoms It contains 6 02 x 1023 C6H6 molecules It has a mass of 78 0 amu It has a mass of carbon that is equal to 6 12 0 72 0 amu

Physical Chemistry
General7 3 a Explain the formation of calcium chloride with the help of an electron dot structure Atomic numbers Ca 20 Cl 17 b Why do ionic compounds not conduct electricity in solid state but conduct electricity in molten and aqueous state Sample Question Paper 2020 21

Physical Chemistry
GeneralFor example if T 4 ms then d 30 cm which gives the scorpion a perfect fix on the beetle EXERCISE A plane progressive wave propagating along positive x axis is 1 y A sin of kox 3 y A sincot sin x 2 y A sin ot kx 4 y A sin of kx

Physical Chemistry
General70 The enthalpy changes for the following processes are listed below Cl g 2C1 g 242 3 kJ mol 1 12 g 21 g 151 0 kJ mol 1 ICI g I g Cl g 211 3 kJ mol 1 62 76 kJ mol 1 1 s 1 g are Given that the standard states for iodine and chlorine 12 s and Cl g the standard enthalpy of formation for ICl g is a 14 6kJ mol 1 c 16 8 kJ mol 1 1 2 4X68 2 1 Oxidicin b 16 8 kJ mol d 244 8 kJ mol 1

Physical Chemistry
GeneralStructure of FXeN SO F 2 Pale yellow solid and FXeOSO F are given below F Xe 1 II F 1 8 Xe F FF Select the correct statement s It is observed that geometry of nitrogen is planar with respect to it surrounding atom O Maximum seven atoms may lie in one plane in structure 1 10 atoms are sp3 hybridized in structure 1 9 d orbitals are involved in bonding in structure 1 S O bond length of II is smaller than S N bond length in 1

Physical Chemistry
GeneralWhich is NOT an alkali metal 21 811 H No K Rb Cs Fr Mg 121712125 Ca Sc Ba Ra Y FIN 31 20 Lithium Li Sodium Na Nb Cesium Cs Cr Hf Ta W Re Os Rf Db Sg Potassium K Periodic Table of the Elements PFA Mn Fe 81115 Bh Hs 31222 Co NI Cu Zn Mo Tc Ru Rh Pd Ag Cd In 123F31 1210 2211131 Sb 0203 Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr S 1 3 E He No Br Kr Sn Pt Au Hg Tl Pb Bi Po At Rn Mt Ds Rg Cn Uut F1 Uup Lv Uus Uuo

Physical Chemistry
General29 The tension in a wire is decreased by 19 The percentage decrease in frequency will be 1 0 19 3 19 2 10 4 0 9

Physical Chemistry
GeneralIdentify the statements which is are incorrect w r t model of an atom Probability density at the nucleus for s and p orbital depends on n n m respectively as per wave mechanical model As per Bohr s theory ground state angular momentum is equal to h 2T The distance of antinode in the radial probability distribution graph of 1s orbital for H Het Li is in the ratio 6 3 2 As per wave mechanical model penetration power as well as angular momentum increases as the value of azimuthal quantum number increases

Physical Chemistry
GeneralPhosphorus exists as P4 has characteristic white color In basic medium it disproportionates as P4 NaOH PH3 NaH PO2 If P Number of P P linkages in P4 Q Number of ionisable H in H3PO2 R Number of species among NH3 H O BF3 CH4 that have angle larger than PH3 S Difference between oxidation states of central atom in products then value of P S R Q 5 is L TETTE M

Physical Chemistry
General6 2 g of a sample containing Na2CO3 NaHCO3 and non volatile inert impurity on gentle heating loses 5 of its weight due to reaction 2NaHCO3 Na2CO3 H O CO Residue is dissolved in water and formed 100 mL solution and its 10 mL portion requires 7 5 mL of 0 2 M aqueous solution of BaCl2 for complete precipitation of carbonates Determine weight in gram of Na2CO3 in the original sample A 1 59 B 1 06

Physical Chemistry
Generalwould be formed 18 A hydrocarbon compound on combustion gives 6 6g of CO and 2 7g of H O and no other products are obtained If 11 35L of gas has mass 14g at STP then find the molecular formula of the gas by calculation hydrogen atom and calcula

Physical Chemistry
GeneralThe picture below shows two cases in which a person is trying to burn the paper cup In case 1 the cup has water in it and in case 2 it is empty and dry Identify in which of the cases the paper will burn Options Case 1 Case 2 Both case 1 and case 2 Water Case 1 Paper cups Case 2

Physical Chemistry
GeneralQuestion Number 148 Question Id 8135612068 Question Type MCQ Display Question Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical Identity the starting material i and product iii in the following So Faro 1 508 500 iii so good 2 R X Options 1 3 1 1 i Br Br Br Na dry ether Na 26 65 Mg dry ether Mg J2 666 Br iii iii ii D D D 0 111

Physical Chemistry
GeneralDuring electro refining of Cu by electrolysis of an aqueous solution of CuSO using copper electrodes if 2 5 g of Cu is deposited at cathode then at anode 1 decrease of more than 2 5 g of mass takes place 2 445 ml of O at STP is liberated 3 2 5 g of copper is deposited 4 a decrease of 2 5 g of mass takes place

Physical Chemistry
GeneralWhich of the following statement s is are correct 1gm of N 0 and 1gm of CO contains same number of atoms 1 litre of 20 w v solution of NaOH and 1 3 kg of 5m urea solution contains same mass of solute If equal molecules of H and O2 reacts to form H O2 then H will be limiting reagent Ferric sulphate Fe2 SO4 3 contains 28 by mass of iron Fe 56 S 32 O 16

Physical Chemistry
GeneralFor the reaction 1 2 2SO2 g O2 g Pt2SO3 g r K SO SO3 2 which of the following is incorrect 1 The overall order of reaction is 1 2 2 The reaction slows down as the product SO3 builds up 3 The order of reaction with respect to SO is 1 4 Unit of rate constant is L mol sec

Physical Chemistry
Generalx mol of oxalate FeC2O4 Fe2 C2O4 3 2H O on reaction with Al2 Cr2O7 3 requires 500 ml 0 4M of it Select the correct statement s n factor of Al2 Cr O7 3 is 6 On factor of Al2 Cr2O7 3 is 18 Moles of oxalate which react with Al2 Cr2O7 3 is 0 4 Moles of oxalate which react with Al2 Cr O7 3 is 0 65

Physical Chemistry
GeneralConsider the following series of reactions 1 NH3 O2 Yield 100 NO H O II NO O Yield 100 NO2 III NO H O Yield 100 HNO3 HNO2 IV HNO Yield 60 HNO3 NO H O 2 moles of NH3 is reacted with 68 1 L of O at STP in a vessel according to the above series of reactions Find volume of NO pro in L at STP

Physical Chemistry
GeneralQuestion Number 144 Question Id 8135612064 Question Type MCQ Display Questi Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical An isomer of propanol among the following is sodat de Jus su st Options Ethyl methyl ether 1 Doe da 2 Diethyl ether Acetone 3 Dess

Physical Chemistry
GeneralAP EAMCET 2020 Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical A hydrocarbon C6H 0 absorbs only one molecule of H upon catalytic hydrogenation Upon ozonolysis the hydrocarbon gave hexane dial Identify the hydrocarbon howsoevod Jebs dojes PESOS CH 0 Pobsone as ws H m Options 1 Cyclohexane 2635 Benzene 2 BodS Cyclohexene 3 2635 Cyclobutene jo eg Food

Physical Chemistry
GeneralComplex A has a composition of H 2O6Cl Cr If the 12 complex on treatment with conc H SO4 loses 13 5 of its original mass the correct molecular formula of Ais JEE Main 2020 Given atomic mass of Cr 52 amu and CI 35 amu 1 Cr H O JCI 2 Cr H O CI JCI 2H O 3 Cr H O Cl 3H O 4 Cr H O CIJCI H O

Physical Chemistry
GeneralNumber Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical In the equation KBr03 5KBr 6HNO3 the amount of KBr03 required in moles to produce 0 07 moles of Br Options 1 0 22 2 0 02 AP EAMCET 2020 KBr03 5KBr 6HNO3 6KNO3 3Br2 3H 0 woes 0 07 e Br Satrones D Breve KBrO3 do 3 0 07 AP EAMCET 2020 4 0 21 6KNO3 3Br2 3H 0 Calculate

Physical Chemistry
GeneralConsider the following redox reaction Cu S s MnO aq acidic medium Cu aq SO2 aq Mn aq The number of moles of MnO4 ion that will be needed to oxidize one mole of Cu S completely is GIN

Physical Chemistry
GeneralAP EAMCET 2020 Two moles of compound A on treatment with a strong base gives two compounds B and C The compound B on dehydrogenation with Cu gives A while acidification of C gives a carboxylic acid D having empirical formula CH O Identify A and D dores A 635 von as wedians rodn do Jobsyds B suatu C y tres boy255 8 2 Cudd gabson adsor sen A Dojd se C usebyson doe CH 0 er der de D do sou boyda 500 A ubat D exto rogodiod Options HCHO HCOOH z HCHO CH CH CUL CUO 6 UCOou

Physical Chemistry
GeneralNegative Marks 40 gm mixture of N and H is taken in stoichiometric ratio and gives ammonia with 20 yield The produced mass of ammon ram is x Then the value of 17 is

Physical Chemistry
GeneralQuestion Number 145 Question Id 8135612225 Question Type MCQ Display Question Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical Calculate the mass of 0 5 mole of ozone molecules 0 5 Breve she dood Options 1 8g 2 16 g 3 24 g 4g

Physical Chemistry
Generalmothongo Arrange the following metal complex compounds in the increasing order of spin only magnetic moment Presume all the three high spin system Atomic numbers Ce 58 Gd 64 and Eu63 mathongo a NH4 2 Ce NO3 6 mathongo b Gd NO3 3 and c Eu NO3 3 mathongo mathongo Answer is mathongo 7 mathongo mathongo mathongs mathongo 1 b a c 2 c a b 3 a b c 4 a c b mathongo mathongo mathongo mathongo mathongo mathongo mathongo mathongo mathong mothonun mathonmathondo mathongo mathonou mathongo mathonco mathant

Physical Chemistry
Generalons ES of oxygen is 023 023 is approx 10 Sum of number of protons electrons and neutr 12 in 12g of C is 1 1 8 3 1 084 x 1025 11 The weight of one atom Its actual weight is g 2 12 044 x 1023 4 10 84 10 23 of Uranium is 238 121 3 91 x 10 22 Which contains 1 1 g CO 3 1 g 02 21 If V mL of the W g Then ma 1 W V x 25

Physical Chemistry
GeneralQuestion Number 156 Question Id 8135611756 Question Type MCQ Display Quest Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical A 1000 Watt bulb emits a monochromatic light of wavelength 400 nm Calculate the number of photons emitted per second by the bulb 1000 Jeso 400 nm e Joongo de D5 50 5000 0 B Je Dod as sve davaty Jerz Donj nedowod Options 1 2 3 2 01 x 1020 S 1 20 12 x 1020 S 1 10 06 x 1020 S 1 AP EAMCET 2020 AP EAMCET 2020

Physical Chemistry
GeneralThe chemical formula for barium sulfide is Bas A chemist determined by measurements that 0 070 moles of barium sulfide participate in a chemical reaction Calculate the mass of barium sulfide that participates Be sure your answer has the correct number of significant digits m me

Physical Chemistry
GeneralConsider the given statements w r t reaction of primary aliphatic amines with NaNO and HCI SI It liberate N gas and forms alcohol SII A carbocation is formed due to evolution of N which can rearrange to form a more stable carbocation SIII The quantitative evolution of nitrogen is used in estimation of amino acids and protein The correct statements among these are Only one correct answer A St and Sill only B Sl and Sll only

Physical Chemistry
GeneralQuestion Number 129 Question Id 8135612209 Question Type MCQ Display Ques Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical 500 ml of gas A at 800 torr pressure and 1000 ml of gas B at 600 torr pressure are placed in a 2 L vessel Calculate the total pressure of the gas mixture in the vessel 800 torr ero Sas de 500 ml e A Jou 38 1000 ml e B Jawe as 21 ge tour tg Options 1 0 5 atm 2 1 0 atm 3 0 1 atm 0 6 atm 600 torr Sassu ne Jawb o Saso Dod

Physical Chemistry
GeneralQuestion Number 131 Question Id 8135611731 Question Type MCQ Display Quest Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical MnO KOH MnO KOH Options 1 Mn304 2 KMnO4 3 K MnO4 4 Mn OH Air mo

Physical Chemistry
GeneralQuestion Number 143 Question Id 8135611743 Question Type MCQ Display Question Number Yes Is Question Mandatory No Single Line Question Option No Option Orientation Vertical Which among the following shows most affinity towards haemoglobin Soders De vjoo uide 0of Towes susy 3 arba da Options 1 CO 2 NO 3 CO