Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
IsomerismHow many 5d orbitals are there in an atom?
What is the maximum number of electrons possible in a set of 5d orbitals?
Organic Chemistry
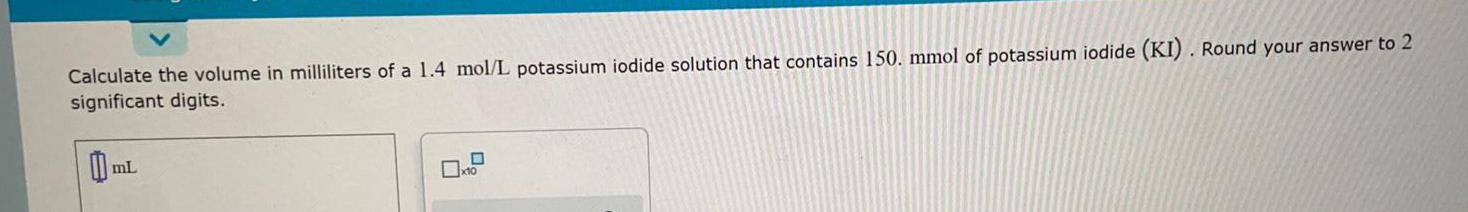
Practical DetectionCalculate the volume in milliliters of a 1.4 mol/L potassium iodide solution that contains 150. mmol of potassium iodide (KI). Round your answer to 2 significant digits.
![A sample of 0.0084 mol of HCI is dissolved in water to make a 1500 mL solution. Calculate the molarity of the HCI solution, the [H3O+] and the pH. For a strong acid such as HCI, the [H3O+] is the same as the molarity of the HCI solution.
HCl(aq) + H₂O (l)→ H3O+ (aq) + Cr (aq)](https://media.kunduz.com/media/sug-question/raw/58551056-1659561614.7168345.jpeg?w=256)
Organic Chemistry
Chemistry in Daily LifeA sample of 0.0084 mol of HCI is dissolved in water to make a 1500 mL solution. Calculate the molarity of the HCI solution, the [H3O+] and the pH. For a strong acid such as HCI, the [H3O+] is the same as the molarity of the HCI solution.
HCl(aq) + H₂O (l)→ H3O+ (aq) + Cr (aq)

Organic Chemistry
General organic chemistryWhat volume of hydrogen gas is produced when 27.8 g of sodium reacts completely according to the following reaction at 25 °C and 1 atm?
sodium (s) + water(l) -> sodium hydroxide (aq) + hydrogen(g)
liters hydrogen gas

Organic Chemistry
General organic chemistryIn a flask, 10.3 g of aluminum reacted with 100.0 g of liquid bromine to form aluminum bromide. After the reaction, no aluminum remained and 8.5 grams of bromine remained unreacted. How many grams of bromine reacted? How many grams of compound were formed?

Organic Chemistry
General organic chemistryWhat volume of hydrogen gas is produced when 77.4 g of iron reacts completely according to the following reaction at 25 °C and 1 atm?
iron (s) + hydrochloric acid(aq) -> iron(II) chloride (aq) + hydrogen(g)

Organic Chemistry
General organic chemistryStudy "Gerunds as Objects of Preposition" on page 147 first.
Which of these sentences with a gerund correct?
A) You have succeeded learning grammar.
B) You should take advantage in working with a tutor.
C) Are you afraid of do yoga?
D) I am interested in learning more about meditating.

Organic Chemistry
Chemistry in Daily LifeWrite the full electron configuration (1s²2s², etc.) for each of the following elements.
a. magnesium, Z = 12
b. carbon, Z=6

Organic Chemistry
BiomoleculesComplete the following vocabulary exercise relating to the level of structure in proteins.
Match the words in the left-hand column with the appropriate blank in the sentences in the right-hand column.
Primary
Secondary
Tertiary
Quaternary
structure is achieved when a protein folds into a compact, three-dimensional shape
stabilized by interactions between side-chain R groups of amino acids
structure is the sequence of amino acids in a protein.
structure is the result of two or more protein subunits assembling to form a larger,
biologically active protein complex.
structure describes the alpha-helices and beta-sheets that are formed by hydrogen
bonding between backbone atoms located near each other in the polypeptide chain.

Organic Chemistry
General organic chemistryWhich of these sentences contains a GERUND?
A) I am not sleeping well right now.
B) Sleeping with a light on isn't a great idea.
C) I have been sleeping much better lately.
D) It's important to sleep in a dark room.
![Give the electron configurations for O and Ir using the short-hand, noble gas method. For example, the electron configuration 1s22s22p63s23p2 (for silicon) is [Ne]3s2 3p².
HOW DO WE GET THERE?
Give the electron configuration for O using the short hand, noble gas method.](https://media.kunduz.com/media/sug-question/raw/58592274-1659560284.102023.jpeg?w=256)
Organic Chemistry
General organic chemistryGive the electron configurations for O and Ir using the short-hand, noble gas method. For example, the electron configuration 1s22s22p63s23p2 (for silicon) is [Ne]3s2 3p².
HOW DO WE GET THERE?
Give the electron configuration for O using the short hand, noble gas method.

Organic Chemistry
IsomerismWhat is a gerund?
A) Acts as a noun in a sentence
B) Acts as a continuous tense in a sentence
C) is formed by "verb-ing"
D) Can be the subject or the object in the sentence
E) A and B
F) B and C
G) A, C, and D

Organic Chemistry
Chemistry in Daily LifeRemember entering vary large or very small
numbers:
• For very large numbers: example 3 x 108 enter
it as 3E8
• For very small numbers: example 6.626 x 10-34
enter it as 6.626E-34
Visible light is a form of Select an answer
Electromagnetic radiation is energy transported in
the form of Select an answer
The only type of electromagnetic radiation we can
see is Select an answer

Organic Chemistry
General organic chemistrySort the agents according to the way they disrupt the structure of proteins: denaturation or hydrolysis.
Drag each item to the appropriate bin.
View Available Hint(s)
Denaturation
mechanical agitation weak acid or base heavy metal ions heat isopropyl alcohol pepsin or trypsin
Hydrolysis
Organic Chemistry
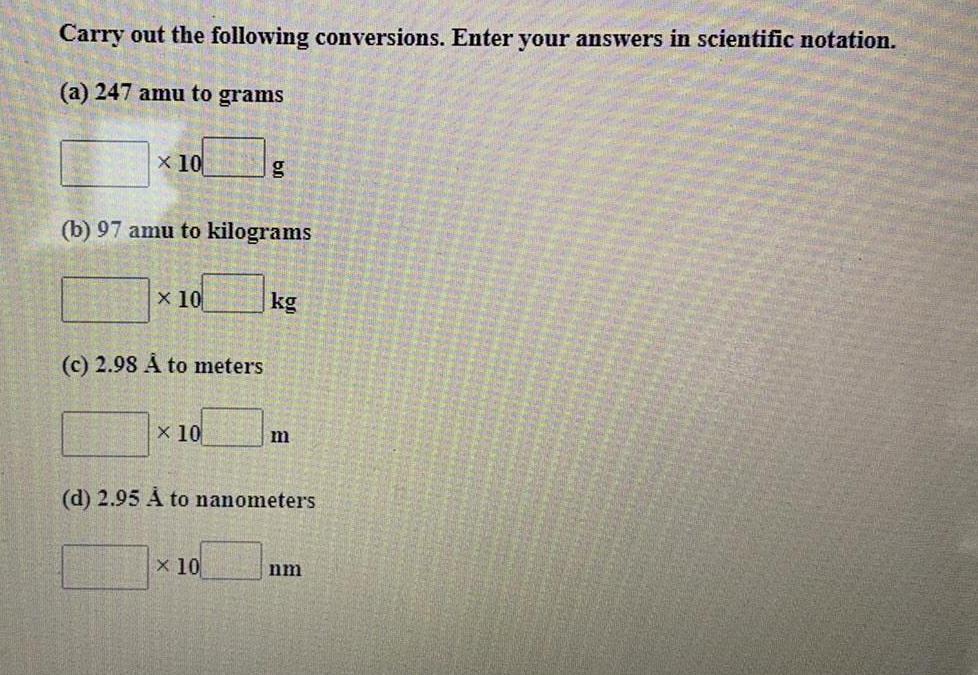
Practical DetectionCarry out the following conversions. Enter your answers in scientific notation.
(a) 247 amu to grams
(b) 97 amu to kilograms
(c) 2.98 A to meters
(d) 2.95 À to nanometers

Organic Chemistry
General organic chemistryWhich of the following statements are true with respect to enzyme activity?
Check all that apply.
Enzymes speed up the reaction rate.
Enzyme reactivity is not affected by change in pH and temperature.
The activation energy of a reaction increases when an enzyme is used to catalyze the reaction.
Enzymes affect the reaction pathway by forming an enzyme-substrate complex.
Enzymes are regenerated when the reaction is completed.

Organic Chemistry
General organic chemistryYou have added an irreversible inhibitor to a sample of enzyme and substrate. At this point, the reaction has stopped completely. What is the best way to get the activity of the enzyme back up?
Removing the irreversible inhibitor should get the reaction working again.
The enzyme is inactive at this point. Nothing can be done except add new enzyme.
Adding more substrate will increase the rate of reaction.
Adding more inhibitor should get the reaction up to speed again.

Organic Chemistry
General organic chemistryIn determining the density of a rectangular metal bar, a student made the following measurements: length, 8.53 cm; width, 2.4 cm; height, 1.0 cm; mass, 52.7064 g. Calculate the density of the metal to the correct number of significant figures.

Organic Chemistry
General organic chemistryIn terms of electrons, what is an excited state? Give an example of an electron configuration
for an excited state.
An excited state is when an electron temporarily occupies an energy state greater than its
ground state.

Organic Chemistry
General organic chemistryClassify the solutes in each of the following equations as a weak electrolyte, a strong electrolyte, or a nonelectrolyte in water:
a. XY₂ (s) → X²+ (aq) + 2Y (aq)
b. HX (g)
XYZ (s) → XYZ (aq)
C.
d. YOH (s)→Y (aq) + OH(aq)
H(aq) + X¯ (aq)

Organic Chemistry
General organic chemistry(R)-6-iodo-3-isopropylnon-1-ene
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars. Show the app
the dashed or wedged buttons and then clicking a bond on the canvas.

Organic Chemistry
General organic chemistryGiven the following equation: 8 Fe + S8 → 8 FeS
What mass of iron, Fe, is needed to react with 16.0 moles of sulfur, S8?
How many moles of iron, Fe, are reacted to form 16.0 grams of FeS (87.91 g/mol)?

Organic Chemistry
General organic chemistryWhich of the following is not equal to 120 centimeters?
0.0012 kilometers (km)
0.012 dekameters (dkm)
1200 millimeters (mm)
1.2 meters (m)

Organic Chemistry
Chemistry in Daily LifeWhich of the following is NOT a useful tip in balancing chemical equations?
Change the formula of the compounds so that the atoms balance on the reactants and products side of the equation.
Balance polyatomic ions as single entities if they appear on both sides of the equation.
Start with all other elements other than hydrogen and oxygen.
If there are an even number of atoms on one side and an odd number of atoms on the other side begin by multiplying the odds by 2.

Organic Chemistry
General organic chemistryMelting point can be defined as the temperature when a solid becomes a liquid. The melting point of the chemical methane is -182°C.
Which state of matter would you expect to exist for methane at a temperature of -181°C?
gas
plasma
liquid
solid

Organic Chemistry
General organic chemistryMr. Fluffinutter is working on his sicence experiments and calculates the molar mass of P2O5 to be 46.973 g/mol to check his answer he asks google the molar mass of P2O5 Google tells Mr. Fluffinutter that the molar mass of P2O5 is 283.886 g/mol. Both google
and Mr. Fluffinutter have failed to calculate the correct molar mass of of P2O5.
Explain what Mr. Fluffinutter did wrong in his calculation, what is the correct molar mass of P205 ?

Organic Chemistry
General organic chemistryBased on your lab report, provide the chemical reaction that occurs in Benedict's test for the following molecule. (Hint: Concentrate on the head of the molecule). ---- Files uploaded sideways or upside down may not be graded.

Organic Chemistry
BiomoleculesIn an a helix, how does bonding occur between the amino acids in the polypeptide chain?
Match the words in the left column to the appropriate blanks in the sentences on the right.
NH group
in a woven string
R group
in the next turn of the spiral
along parallel sheets
C=O group
Request Appor
In the alpha helix, there is hydrogen bonding between the
of another amino acid
Reset Help
of one amino acid and the
of the polypeptide chain.

Organic Chemistry
Chemistry in Daily LifeMr. Bean is performing an experiment in the laboratory and the procedure calls for a 2.00 M solution of HCI. Mr. Bean has a 11.9 M solution of HCI. So Mr. Bean decides he needs to make a dilution. SO Mr. Bean dissolves 0.0420 L of the 11.9 M HCL to a total volume of 500 mL. Did this give Mr. Bean a 2.00 M solution of HCI? If not how should have Mr. Bean prepared the solution?

Organic Chemistry
General organic chemistryA saline solution used in intravenous drips for patients who cannot take oral fluids contains 0.92% (w/v) NaCl in water. How many grams of NaCl are contained in 5755 mL of this solution?
A. 5.3 g NaCl
B. 1.6 g NaCl
C. 529 g NaCl
D. 0.016 g NaCl
E. 53 g NaCl

Organic Chemistry
General organic chemistryGive the major substitution product of the
following reaction.
CH3CH₂Br
CH3OH
CH3CH₂OH
CH3CH2NH2
CH3CH₂O₂CCH3
CH3CH₂SCH 3
CH3CH₂CH3
There is no reaction under these
conditions or the correct product is not
listed here.

Organic Chemistry
Alcohols and PhenolsWhich of the following statements is true?
Multiple Choice
Saliva contains keratin, a slippery protein that is used to make mucus.
Insulin and glucagon are proteins.
Most human cells rely almost entirely on protein for energy.
Edema occurs when a healthy person consumes more protein than needed.

Organic Chemistry
General organic chemistryWhich one of the following is not equal to 100 meters?
10,000 centimeters
10 hectometers
100,000 millimeters
0.100 kilometers

Organic Chemistry
General organic chemistry1. Section Question: What do elements in Period
3 all have in common?
Their atoms have the same number of
valence electrons.
Their atoms have the same number of
electron shells.
Their atoms have the same properties.
None of the above.

Organic Chemistry
General organic chemistryA weather balloon has a volume of 3.9 L at ground level, where the pressure is 1.0 atm. What would be the volume of the balloon once the pressure drops to 0.88 atm in the troposphere? (assume all other variables remain the same)

Organic Chemistry
Chemistry in Daily LifeLiquid octane (CH₂(CH₂) CH₂) reacts with gaseous oxygen gas (0₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). If 4.18 g of water is produced from the reaction of 8.00 g of octane and 12.9 g of oxygen gas, calculate the percent yield of water.
Round your answer to 3 significant figures.

Organic Chemistry
Isomerism(a) Construct a table of reagents, with columns for amount used, molecular weight, moles, and
equivalents (you may need another column for density In some cases).
(b) Calculate the percent yield.
Please show ALL your work, and be sure to pay attention to SIGNIFICANT figures. Fallure to do elther will result in a loss of points.
(2) 4.4895g of the alkyl halide was reacted with 7.13g of the cyanide salt to give 1.3558 of product.

Organic Chemistry
Practical DetectionIf the flask is open to the atmosphere, the mercury levels are equal. For each of the following situations where a gas is contained in the flask, calculate the pressure in the flask in torr,
atmospheres, and pascals.
with atmospheric P= 652 torr, height in tube 140. mm greater on side of flask

Organic Chemistry
AminesA real gas behaves more like an ideal gas when the gas molecules are
1. close and have strong attractive forces between them
2. close and have weak attractive forces between them
3. far apart and have strong attractive forces between them
4. far apart and have weak attractive forces between them

Organic Chemistry
Chemistry in Daily LifeCalcium oxide can be used to "scrub" carbon dioxide from air.
CaO(s) + CO₂(g) → CaCO3(s)
What mass of CO₂ could be absorbed by 1.75 g of CaO?
Mass= 1.375 g CO₂
What volume would this CO₂ occupy at STP?

Organic Chemistry
General organic chemistryA 0.734-g sample of an unknown gas has a volume of 739 mL. and a pressure of 569 mmHg at 31.7 °C, Calculate the molar mass of this compound.

Organic Chemistry
BiomoleculesComplete the general structure of an L-a-amino acid by assigning groups to the correct positions.
This L-alpha amino acid should be drawn at physiological pH. Be sure to include formal charges. Use R for the side chain.

Organic Chemistry
IsomerismGaseous ethane (CH3CH3) reacts with gaseous oxygen gas (O₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). If 0.408 g of water is produced from the reaction of 0.60 g of ethane and 1.3 g of oxygen gas, calculate the percent yield of water.
Round your answer to 2 significant figures.

Organic Chemistry
General organic chemistryDraw all resonance structures of the following
carbanion and carbocation. Be sure to include the
initial structure in your answer as well.
Draw the molecules on the canvas by choosing buttons from the Tools (for bonds and charges), A
toolbars.
![In a particular experiment at 300 °C, [NO2] drops from 0.0270 to 0.00821 M in 268 s. The rate of disappearance of NO₂ for this period is _ M/s.
1.40×10-4
-1.31x10-4
1.43x104
3.51x10-5
7.01x10-5](https://media.kunduz.com/media/sug-question/raw/58058407-1659558832.1897001.jpeg?w=256)
Organic Chemistry
General organic chemistryIn a particular experiment at 300 °C, [NO2] drops from 0.0270 to 0.00821 M in 268 s. The rate of disappearance of NO₂ for this period is _ M/s.
1.40×10-4
-1.31x10-4
1.43x104
3.51x10-5
7.01x10-5

Organic Chemistry
General organic chemistryA balloon holds 39.8 kg of helium. What is the volume of the balloon if the pressure is 1.25 atm and the temperature is 25 °C?


Organic Chemistry
General organic chemistryDraw a Lewis structure for nitrogen pentoxide (N₂O5) in which each N is bonded to three O atoms. Be sure to include all lone pair electrons and nonzero formal charges. (Hint: None of the oxygen atoms are bonded to each other.)

Organic Chemistry
Practical DetectionThe measured dipole moment of hydrobromic acid, HBr, is 0.82 D and the H-Br bond distance is
1.41 Å. Determine the percent ionic character of the bond in HBr.

Organic Chemistry
General organic chemistryA 1.80 gram sample of helium gas has a volume of 933 milliliters at a pressure of 3.77 atm. The temperature of the He gas sample is