Organic Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Organic Chemistry
Practical DetectionHow many grams of Ti are there in a sample of Ti that contains 4.06×1023 atoms?

Organic Chemistry
General organic chemistryDetermine the concentrations of MgCl₂, Mg2+, and CI in a solution prepared by dissolving 2.43 x 10 g MgCl₂ in 2,25 L of water. Express all three concentrations in molarity. Additionally, express the concentrations of the ionic species in parts per million (ppm).

Organic Chemistry
General organic chemistryHow many ATOMS of carbon are present in 5.28 grams of carbon tetrabromide ? 9.58x10^21 2. How many GRAMS of bromine are present in 8.91x1022 molecules of carbon tetrabromide ?

Organic Chemistry
General organic chemistryParts per million (ppm) is a common way to express small concentrations of a solute in water. A sample of tap water that is 25 ppm Cl contains 25 grams of CI for every 1,000,000 grams of water. Which units are numerically equal to ppm for dilute aqueous solutions?
g/L
mg/L.
cg/L.
µg/L
ng/L

Organic Chemistry
Practical DetectionArticle: How Soddy's nightmare about the future of radioactive isotopes realized?
The use of radioactive isotopes to quickly end World War II.
The use of radioactive isotopes to provide energy.
The use of radioactive isotopes to model climate change.
The use of radioactive isotopes to be used in medicine.
Organic Chemistry
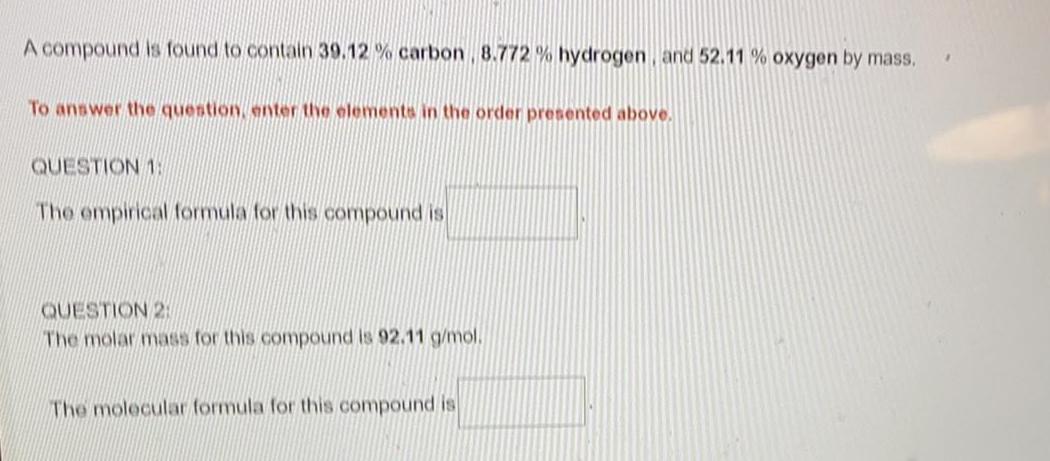
General organic chemistryA compound is found to contain 39.12% carbon, 8.772 % hydrogen, and 52.11 % oxygen by mass.
The empirical formula for this compound is
The molar mass for this compound is 92.11 g/mol.
The molecular formula for this compound is

Organic Chemistry
General organic chemistryCalculate the mass in grams for each of the following samples.
a. 1.08 moles of aluminum chloride
b. 3.62 moles of sodium hydrogen carbonate
c. 4.21 millimoles of hydrogen bromide (1 millimole = 1/1000 mole)

Organic Chemistry
General organic chemistryWhat would you multiply "atoms of cesium" by to get the units "moles of cesium"?

Organic Chemistry
General organic chemistryWhat would you multiply "moles of cesium" by to get the units "grams of cesium"?
Drag and drop your selection from the following list to complete the answer:

Organic Chemistry
General organic chemistryA cat with a mass of 5 kg is sitting on a skateboard. A rat with a mass of 0.5 kg is sitting on an identical skateboard. 2. If you apply the same force to push each skateboard, will the cat or the rat accelerate the fastest? Explain.

Organic Chemistry
Aldehydes & KetonesThe 2009 government bailout of automotive companies was an example of
The expressed powers of the Federal Government
The implied powers of the Federal Government
The implied powers of State governments
The expressed powers of State governments

Organic Chemistry
General organic chemistryWhich of the following actions would cause the rate of the reaction below to decrease?
CuSO4 + Mg MgSO4 + Cu
a. Heating the reaction.
b. Adding magnesium.
c. Removing copper.
d. Adding a catalyst.
e. Cooling the reaction.

Organic Chemistry
General organic chemistryIf 17.4 grams of hydrochloric acid (HCI) reacted, how many grams of hydrogen (H₂) were produced? NOTE: HCI has a molar mass of 36.46g and H₂ has a molar mass of 2.02g.

Organic Chemistry
General organic chemistryThe hydrogen deficiency index (HDI) of C4H100 is, which means this compound has pi bonds or rings or combination of pi bonds and rings.
2
3
Clear selectic

Organic Chemistry
BiomoleculesWhat monosaccharide is different than all the others at the top of the structures?

Organic Chemistry
BiomoleculesWe saw in this activity that oxidation reactions of alcohol groups in carbohydrates can
provide enough energy to transfer electrons to NAD+.
a. Which form of this cofactor is the more oxidized form-NAD* or NADH?
b. Which form is the more reduced form?
c. Given that in an oxygen-containing
environment, oxidation reactions are
generally favorable (spontaneous), which form (oxidized or reduced) is at a higher potential energy level?
d. In general, would oxidized cofactors or reduced cofactors provide a "stockpile" of energy? Explain.

Organic Chemistry
AminesLearning Goal: In the presence of a strong acid, such as HCl, an amide reacts with water to produce a carboxylic acid and an ammonium or amine salt. In the presence of a strong base, like NaOH, the amide reacts to produce ammonia or an amine, and a carboxylic acid salt. Acetaminophen is an analgesic marketed under the brand name Tylenol, among others. (Figure 1) Draw the amine that results from the base hydrolysis of acetaminophen.

Organic Chemistry
General organic chemistryA 1.5g sample of coniine (shown below) was dissolved in 10 mL of ethanol and placed in a cell with a 5.0 cm path length. The observed rotation was +1.21°. Calculate the specific rotation of coniine (2 pts). Is ethanol an acceptable solvent to use?

Organic Chemistry
General organic chemistryDraw the expanded structural formula of two water molecules and indicate how each of these forms hydrogen bonds with the molecule shown below. Use dotted lines to indicate hydrogen bonds. --- Files uploaded sideways or upside down will not be graded.


Organic Chemistry
IsomerismWhich of the following ionic compounds is soluble in water?
a (NH4)2S
b. Cas
c. Cu₂S
d. Al2S3
e. MgS

Organic Chemistry
Chemistry in Daily LifeAn aqueous solution of potassium chloride is mixed with an aqueous solution of sodi
Identify the solid in the balanced equation.
a. NaCl
b. KCI
c. There is no solid formed when the two solutions are mixed.
d. NaNO3
e. KNO3

Organic Chemistry
Aldehydes & KetonesWhich of the following is the best example of a comma splice?
A. Using a comma with a coordinating conjunction to make a complex or compound sentence
B. Using a comma incorrectly
C. Using two commas when only one is needed
D. Using a comma to join independent clauses that could stand alone as sentences

Organic Chemistry
Chemistry in Daily LifeAt 448°C, the equilibrium constant for the reaction
is 50.5. What concentration of
12 would be found in an equilibrium mixture in which the concentrations of
H₂ were 0.450 M and 0.045 M, respectively?

Organic Chemistry
General organic chemistryLaurediol is a compound isolated from marine algae. Designate sequentially (1,2,3,4) the stereochemistry at the numbered sites in the given drawing of Laurediol.

Organic Chemistry
General organic chemistryThe e.m.f of cell in V: H₂(g) |Buffer || Normal caloal electrode is 0.6885 V at 40°C when the barometric pressure is 725 mm of Hg. What is the pH of the solution. E = 0.28 .(write the value to the nearest integer)

Organic Chemistry
Chemistry in Daily LifeDoes a reaction occur when aqueous solutions of chromium(II) sulfate and barium chloride are combined?
If a reaction does occur, write the net ionic equation.
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Be sure to specify states such as (aq) or (s).

Organic Chemistry
General organic chemistryHow many mL of 0.75 M H3PO4 would be required to titrate 200.0 mL of 0.50 M Ca(OH)2 to
the equivalence point? (4 points) The balanced reaction has been provided.

Organic Chemistry
Chemistry in Daily LifeMany chromate (CrO4) salts are insoluble, and most have brilliant colors that have led to their being used as pigments. Choose the correct net ionic equation for the reaction of Co³+ with a chromate ion.
Co³+ (aq) + CrO4 (aq) → CoC-O4(s)
2Co³+ (aq) + 3CrO4 (aq) → Co₂ (CrO4)3 (8)
2Co³+ (aq) + CrO4 (aq) → Co₂ (C-04)3(S)
2Co³+ (aq) + 3CrO4 (aq) → Co₂ (CrO4)3 (29)

Organic Chemistry
Chemistry in Daily LifeConsider the following compounds. Which is insoluble?
Fel2
PbCl₂
LiBr
AlBr3
All of these
None of these

Organic Chemistry
General organic chemistryConsider the following reaction:
2C₂H6 + 702 4CO2 + 6H₂O
Identify what main category of reaction it is. If possible, further categorize it into all other relevant
types of reaction.
Synthesis
Decomposition
Combustion
Single Replacement
Double Replacement
Precipitation
Acid-Base
Oxidation-Reduction

Organic Chemistry
General organic chemistryConsider the following reaction:
K₂SO4 + Ba(NO3)2 → 2KNO3 + BaSO4
Identify what main category of reaction it is. If possible, further categorize it into all other relevant types of reaction.
Synthesis
Decomposition
Combustion
Single Replacement
Double Replacement
Precipitation
Acid-Base
Oxidation-Reduction
Gas Evolving


Organic Chemistry
General organic chemistryBalance the equation
Fe2S3(s)→ Fe(s) + S(s) + 69kJ
1b) What is the mole to mole ratio of Fe₂S3(s) to Fe(s)
1c.) What is molar mass of Fe2S3(s)
ld.) How many moles is 28 gram of Fe2S3(s)?
le. How many moles of Fe(s) does this produce using answers 1b and 1d.

Organic Chemistry
BiomoleculesA gene on a chromosome lies close to another gene controlling a different trait. This indicates that the genes are first gene contributes to the expression of the second gene. This indicates

Organic Chemistry
General organic chemistryDoes a reaction occur when aqueous solutions of potassium sulfide and nickel(II) nitrate are combined?
If a reaction does occur, write the net ionic equation.
Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.
Be sure to specify states such as (aq) or (s).

Organic Chemistry
General organic chemistryAssume that 6600 NaOH units were dissolved in a sample of water. How many units of Nations does the solution contain? How many units of OH ions does the solution contain?
Organic Chemistry
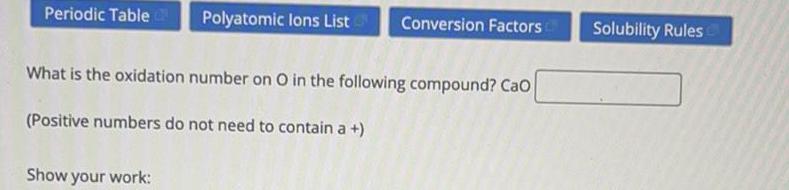
Chemistry in Daily LifeWhat is the oxidation number on O in the following compound? Cao
(Positive numbers do not need to contain a +)
Solubility Rules

Organic Chemistry
IsomerismFor E-stilbene, draw the products you get if the bromine mechanism is syn addition (addition to the same side) and also draw the products you get if the mechanism is anti addition (addition to opposite sides). nexelloko

Organic Chemistry
Chemistry in Daily LifeBalance the following equation and predict the states:
• Remeber to enter 1's for balancing
• States of matter options: s, I, aq, g

Organic Chemistry
Practical DetectionMany chromate (CrO₂) salts are insoluble, and most have brilliant colors that have led to their being used as pigments. Choose the correct net ionic equation for the reaction of Sr²+ with a chromate ion.
Sr²+ (aq) + 2CrO4²(aq) → S-(CrO4)2 (s)
Sr²+ (aq) + CrO² (aq) → SrCrO4(s)
2Sr²+ (aq) + CrO₂ (aq) → Sr₂ CrO4(s)
Sr²+ (aq) + CrO4² (aq) → SrCrO₂ (aq)

Organic Chemistry
General organic chemistryWhich definition best describes polygenic traits?
A. traits that are carried on linked genes
B. traits that are controlled by multiple genes
C. traits that affect several features
D. traits that express both alleles of a gene pair

Organic Chemistry
Practical DetectionConsider the following compounds. Which is/are insoluble? Select all that apply.
Ba(OH)₂
NaOH
CuOH
Hg(OH)₂
All of these
None of these

Organic Chemistry
General organic chemistryWhich one of the following is not one of the four "driving forces" that pull reactants to form products during chemical reactions?
Gas evolution
Water formation
Solid formation
Electron transfer
Formation of a product

Organic Chemistry
General organic chemistryBalance the following reaction: (remember to include any 1's on appropriate compounds)
C4H100₂+0₂ -CO₂ + H₂O

Organic Chemistry
Aldehydes & KetonesThe carbonyl group consists of
carbon and oxygen attached by a double bond
a carbon-oxygen-carbon attached by single bonds
carbon and oxygen attached by a triple bond
carbon-oxygen-hydrogen attached by single bonds
carbon and oxygen attached by a single bond

Organic Chemistry
Practical DetectionConsider the following reaction:
Al+3CuCl → AlCl3 + 3Cu
Identify what main category of reaction it is. If possible, further categorize it into all other relevant
types of reaction.
Synthesis
Decomposition
Combustion
Single Replacement
Double Replacement
Precipitation
Acid-Base
Oxidation-Reduction
Gas Evolving
Question Help: Message instructor
Submit Question

Organic Chemistry
Alcohols and PhenolsDuring acid-catalyzed hydration, which of the following alkenes might lead to a rearranged product?
2-methylbut-2-ene
3,3-dimethylbut-1-ene
4-methylpent-1-ene
3-methybut-1-ene
Both 3,3-dimethylbut-1-ene and 3-methybut-1-ene
All undergone carbocation rearrangement.

Organic Chemistry
Halogen DerivativesPredict the product(s) of each of the following reactions. If you expect a racemic mixture, draw both enantiomers.
Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars.

Organic Chemistry
General organic chemistryWhen the hydroboration oxidation of 2-methylbut-2-ene is carried out, the major product of the reaction will be a
tertiary alcohol.
secondary alcohol.
primary alcohol.
mixture of a primary and secondary alcohols.