Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
EquilibriumSome quantity of water is contained in a container as shown in figure As neon is added to this system at constant pressure the amount of liquid water in the vessel A increases C remains same B decreases D changes unpredictably vapour water

Physical Chemistry
GeneralThe concentration of an organic compound in chloroform is 6 15 6 15 g per 100mL of solution A portion of this solution in a 5cm polarimeter tube causes an observed rotation of 1 2 What is the specific rotation of the compound EAMCET 2009 a 12 b 3 9 c 39 d 61 5

Physical Chemistry
General3 21 molecules 4 101 molecules 4 If the weight of metal oxide is x g containing yg of oxygen the equivalent weight of metal will be 2 E 1 E 8x y E 4 E 8 y x X 8 x y y

Physical Chemistry
Energetics9 For which of these reactions will there be AS positive 1 H O g H O 1 2 H g g 2HI g 3 CaCO s CaO s CO 0 4 N g 3H g 2NH g

Physical Chemistry
ElectrochemistryThe standard electrode potentials of Zn Zn Cu Cu and Ag are respectively 0 76 0 34 and 0 8 V The following cells were constructed 1 ZnZn Cu Cu II ZnZn Ag Ag III Cu Cu Ag Ag What is the correct order of E of these cells 1 2 II I III 3 III 4

Physical Chemistry
Equilibrium18 a 8 0 10 b 8 0 10 15 c 8 0 10 17 d 8 0 10 14 139 What is the minimum pH necessary to cause a precipitate of Pb OH Ksp 1 2 x 10 5 to 2 form in a 0 12 M PbCl solution b 10 8 a 12 4 c 12 0 140 Which of the following would increase the solubility of Pb OH d 11 1

Physical Chemistry
Atomic StructureThe number of waves made by a Bohr electron in H atom for one complete revolution in its 3rd orbit are A 1 B 2 C 3 D 4

Physical Chemistry
GeneralNumber of Fe atoms in 100 g Haemoglob contains 0 33 Fe Atomic mass of Fe 5 1 0 035 1023 3 3 5 1023 An organic compound 2 35 4 7 x 10 containing C and H ga

Physical Chemistry
General3 6 025 x 100 molecules of acetic acid are present in 500 ml of its solution The concentration of solution is 1 0 002 M 2 10 2 M 3 0 012 M 4 0 001 M How many litre of oxygen at STP is required to burn

Physical Chemistry
Generald 5 0 x 10 6 38 On adding AlCl3 to water a the ionisation of water increases b the ionisation of water decreases c the ionisation of water remains constant d the ionic product of water increases What it

Physical Chemistry
GeneralSelect the incorrect statement Potassium carbonate on reaction with hot steam gives KOH and CO2 Potassium serperoxide reacts with sulphur t give potassium sulphide Black ash obtained by the conversion of salt cake Na2SO4 a solid residue with 45 of Na CO3 Sodium chloride is slightly hygroscopic

Physical Chemistry
GeneralSodium chloride is soluble in water but not in benzene because in water and AHAH in benzene in benzene in benzene in benzene 1 AH AH 2 AH 3 AH 4 AH AH AH AH Schon Schon in water and AH AH Las in water and AH Schon AH Laming in water and AH AH Lattice sergy

Physical Chemistry
Atomic StructureIf in a photoelectric cell the wavelength of incident light is changed from 4000 A to 3000 A then change in stopping potential will be 1 0 66 V 2 1 03 V 3 0 33 V 4 0 49 V a Pusa Road New Delhi 110005 Ph 011 47623456

Physical Chemistry
General5 1 5 gm of an unknown gas at 127 C occupies the same volume as 9 6 gm ozone gas at 27 C at the same pressure The molar mass of unknown gas is A 10 gm mol C 15 gm mol B 5 gm mol D 20 gm mol

Physical Chemistry
Electrochemistry3 In which of the following cells EMF is greater than Eccell O A Pt H 8 H pH 5 H pH 3 H 8 Pr B Zn s Zn 0 2M Cu 0 1M Cu s C Cr s Cr 0 1 M Cu 0 2M Cu s 3 D Pt H g H pH 4 H pH 6 H g Pt

Physical Chemistry
Solutions29 How many g of dibasic acid mol weight 200 should be present in 100 ml of the aqueous solution to give strength of 0 1 N 1 10 g 3 19 2 2 g 4 20 g

Physical Chemistry
SolutionsWhich among the following statements is false 1 The correct order of osmotic pressure for 0 01 M aqueous solution of each compound is BaCl KCI CH COOH Sucrose 2 The osmotic pressure of a solution is given by the equation MRT where M is the molarity of the solution 3 Raoult s law states that the vapour pressure of a component over a solution is proportional to it s mole fraction 4 Two sucrose solutions of the same molaltiy prepared in different solvents will have the same freezing point depression

Physical Chemistry
GeneralGiven the numbers 161 cm 0 161 cm 0 016 The number of significant figures for the numbers is 1 3 3 and 4 respectively 2 3 4 and 4 respectively 3 3 4 and 5 respectively 4 3 3 and 3 respectively

Physical Chemistry
GeneralMagnetic moments of V Z 23 Cr Z 24 Mn Z 25 are x y z Hence A x y z B C x z y skipped x y z D z y x

Physical Chemistry
Chemical kineticsWhich of the following statements are correct about catalyst at constant temperature A It does not alter AS of the reaction C It alters AS of the reaction B It does not alter AH of the react D none of these

Physical Chemistry
Chemical Bonding1 CH 2 C H If Fe CO the Fe C bond possesses 1 character only 2 Ionic character characters Which is used in the formation of nylon 6 6 1 Sulphurhexa fluoride 3 CO 3 character only 2 Adipic acid 4 Xe 4 Both o anc AAJ KA TOPPER

Physical Chemistry
General1 m n 2 n m 3 2 4 2 How many carbon atoms are present in 0 35 mole of C H O AAJ KA TOPPER Given N 6 023x10 1 1 26x10 carbon atoms 3 1 26x10 carbon atoms Which of the followfou conficiratione 2 1 26 10 carbon atoms 4 1 26 10 carbon atoms forms on e netobortial no play only

Physical Chemistry
General6 gram atom contains Natoms A One mole of sucrose reacts completely with oxygen produces 268 8 litre of carbon dioxide at STP R Amount of oxygen required for reaction is 268 8 litre 7 A In the reaction

Physical Chemistry
Equilibrium150 Consider the following statement and select correct option 1 Ksp of Fe OH 3 in aqueous solution is 3 8 x 10 38 at 298 K The concentration of Fe will increase when H ion concentration decreases II In a mixture of NH4Cl and NH4OH in water a further amount of NH4Cl is added The pH of the mixture will decreases III An aqueous solution of each of the following salts NH 1 HCOOK will be basic acidic respectively a only I is correct c only III is correct b only II is correct d II and III are correct

Physical Chemistry
Atomic StructureWhich of the following statements is correct 1 The electronic configuration of Cr is Ar 3d 4s Atomic No of Cr 24 2 The magnetic quantum number may have a negative value 3 In silver atom 23 electrons have a spin of one type and 24 of the opposite type Atomi of Ag 47 4 All of the above

Physical Chemistry
EquilibriumM HCI 50 cm 0 2 M NaOH 2 57 At 25 C the solubility product of Hg Cl in water is 3 2x 10 17 mol dm 9 What is the solubility of Hg2Cl2 in water at 25 C a 1 2x 10 12 M c 2 10 6 M e 5 2x 10 6 M Kerala CEE 2011 b 3 0x 10 6 M d 1 2x 10 16 M

Physical Chemistry
GeneralWhich of the following statements about chemisorption is not applicable O O O It involves chemical forces between adsorbent and absorbate OIt is irreversible in nature OIt involves high heat of adsorption OIt involves low activation energy

Physical Chemistry
Solutionsn M Which phenomenon occurs when an electric field is applied to a colloidal solution and electrophoresis is prevented by some suitable means Reverse osmosis takes place Electrosmosis takes place Dispersion medium begins to move Dispersion medium becomes stationary

Physical Chemistry
SolutionsA semipermeable membrane separates a 0 2 M glucose solution from a 0 4 M glucose solution The solvent water will flow from the solution to the solution The volume of the 0 4 M solution will 0 4 M 0 2 M decrease 0 2 M 0 4 M decrease 0 2 M 0 4 M increase Click the answer you think is right Do you know the answer

Physical Chemistry
EnergeticsA cylinder filled with a movable piston contains liquid water in equilibrium with water vapour at 25 C Which one of the following operations results in a decrease in the equilibrium vapour pressure 1 Moving piston downward a short distance 2 Removing a small amount of vapour 3 Removing a small amount of the liquid water 4 Dissolving salt in the water

Physical Chemistry
EquilibriumWhich of the following statement s is are correct about the ionic product of water 4 K ionization constant of water Kw ionic product of water 3 pK pKw C at 25 K 1 8 10 4 D ionic product of water at 10 C is 10 14

Physical Chemistry
GeneralThe amount of zinc required to produce 1 12 ml of H at STP on treatment with dilute HCI will be 1 65 g 2 0 065 g 3 32 5 10 g 4 6 5 g

Physical Chemistry
General38 An element X has the following isotopic composition sex 90 57Xx 8 x 2 0 The weighted average atomic mass of the naturally occurring element X is closest to 1 56 14 amu 3 60 amu 100 of 39 2 56 8 amu 4 55 amu and 64 of rynen

Physical Chemistry
GeneralThe number of mole of nitrogen in one litre of air containing 10 nitrogen by volume under standard conditions is 1 0 03 mole 3 0 186 mole 2 2 10 moles 4 4 46 10 3 mole

Physical Chemistry
Atomic Structure8 An organic compound containing C H and N gave the following analysis C 40 H 13 33 N 46 67 Its empirical formula would be 1 CH N 3 C H N 2 CH N 4 C H N

Physical Chemistry
Gaseous and liquid states32 The specific heat of a gas is found to be 0 075 calories at constant volume and its formula wt is 40 The atomicity of the gas would be 1 One 2 Two 4 Four 3 Three Wa

Physical Chemistry
GeneralThe graph of compressibility factor Z vs P for one mode of a real gas is plotted at constant temperature 273 K If the slope of graph at very dZ 1 IS atm 1 the volume of one molecule of real gas in cm is x x 10 22 Find x Take N 6 1023 dp 10 high pressure

Physical Chemistry
SolutionsA certain amount of a metal whose equivalent m is 28 displaces 0 7 L of H at S T P from an hence mass of the element is 1 1 75 g 2 0 875 g 3 3 50 g 4 7 00 g Number of Fe atoms in 100 g Haemoglobin

Physical Chemistry
Chemical BondingAn element E belongs to modern periodic table with atomic number Z If E 2 has highest energy electron in a subshell for which number of total node are half of orbit number The orbital angular momentum of highest energy electron in E is Assume E has the minimum atomic number that follow above condition but Z 6 Zero 2 h 2T 6 h 2T 12 h

Physical Chemistry
GeneralA cylinder contains either ethylene or propylene 12 ml of gas required 54 ml of oxygen for complete combustion The gas is 1 Ethylene 2 Propylene 3 1 1 mixture of two gases 4 1 2 mixture nific found

Physical Chemistry
EquilibriumThe reaction A B C D is studied in a one litre Vessel at 250 C The initial concentration of A was 3n and of B was n After equilibrium was attained then equilibrium concentration of C was found to be equal to equilibrium concentration of B What is the concentration of D at equilibrium 1 12 2 n 3 n 2 3n 4 n 2

Physical Chemistry
Energetics30 H g O g 0 0 H O H O AH X4 Given EH HX Eo o EO HX AH of H O vapour is 2 2xg X 22 X4 4 X 2 2xy x4 31 A cylinder contains either ethylene or propylene 12 ml of gas required 54 ml of oxygen for complete combustion The gas is 1 Ethylene 2 Propylene 3 1 1 mixture of two gases 4 1 2 mixture 32 The specific heat of a gas is found to be 0 075 calories at constant volume and its formula wt is 40 The atomicity of the gas would be 1 One 3 Three 2 Two 4 Four 33 H g O g 0 H g AH for this reaction is 1 Heat of formation of O H 2 Bond energy of O H 3 Heat of combustion of H 4 Zero at all temperatures 34 Energy required to dissociate 4 g of gaseous H into free gaseous atoms is 872 kJ at 25 C The bond energy of H H bond will be 1 8 72 kJ 3 436 kJ 2 4 36 kJ 4 43 6 kJ 35 The dissociation energy of CH g is 360 kcal mol and that of C H g is 620 kcal mot The C C bond energy 1 260 kcal mol 1 3 130 kcal mol 36 The enthalpy of reaction 2 180 kcal molt 4 80 kcal mol 1 8s 41 4p q 5r 2 4p 2q 5r 85 41 3 4p 2q 5r 8s 4t 4 2p q 5r 8s 41 37 Using bond energy data calculate heat of formation of isoprene 5C s 4H 9 H C C CH CH CH Given C H H H C C C C and C s C g respectively as 98 8 kcal 104 kcal 83 kcal 147 kcal 171 kcal 42 1 21 kcal 3 40 kcal 38 In a flask colourless N O is in equilibrium with brown coloured NO At equilibrium when the flask is heated at 100 the brown colour deepens and on cooling it becomes less coloured The change in enthalpy AH for formation of NO is 1 Negative 2 Positive 4 Undefined 3 Zero 39 For which of these reactions will there be AS positive 2 21 kcal 4 50 kcal 1 H O g H O 1 2 H g g 2HI g 3 CaCO s CaO s CO 4 N g 3H g 2NH g 40 For stretched rubber Entropy 1 Increases 2 First increases then decreases 3 Decreases 4 First decreases then increases 41 The least random state of H O is 1 Ice 2 Liquid water 3 Steam 4 Randomness is same in all AS for the reaction MgCO s MgO s CO g 1 Zero 2 ve 3 ve 4 43 The standard entropies of N g H g and NH g are 191 5 130 5 192 6 JK mol The value of AS of formation of ammonia is

Physical Chemistry
Chemical kineticsThe pH at the second equivalent point of 30 ml of 0 124 M H3PO3 K 2 2 6 107 is tritated with 0 1 M NaOH solution is Given log 26 1 415 log 35 6 1 5514 A 10 57 B 11 57 C 9 57 D 12 57

Physical Chemistry
General3 40 kcal 4 76 kcal 18 The difference between AH and AE for the reaction 12CO g 6H O at 25 C 2 3 72 kJ 2CH 150 0 in kJ is 1 7 43 kJ 31 372k1

Physical Chemistry
Chemical BondingWhen acetylene is passed through 2 points a solution of copper chloride a red precipitate of copper acetylide Cu2C2 is formed Determine the bond order of C2 2 and identify the HOMO and LUMO orbitals Compare the same with C2

Physical Chemistry
Chemical BondingS and Pauli s principles d None of these 119 A compound of vanadium has a magnetic moment u of 1 73 BM If the vanadium ion in compound is present as V then the value of x is a 1 b 2 c 3 120 d6 configuration will result in total spin of d 4
Physical Chemistry
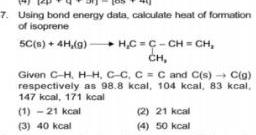
General7 Using bond energy data calculate heat of formation of isoprene 5C s 4H g H C C CH CH CH Given C H H H C C C C and C s C g respectively as 98 8 kcal 104 kcal 83 kcal 147 kcal 171 kcal 1 21 kcal 3 40 kcal 2 21 kcal 4 50 kcal

Physical Chemistry
Gaseous and liquid statesComplete the following table for an ideal gas V IST P 16 628 10 n Temper

Physical Chemistry
GeneralHydroxyl amine reduces iron III according to following equation NH OH Fe SO4 3 N g H O FeSO4 H SO4 Which statement is correct A n factor for Hydroxyl amine is 1 B equivalent weight of Fe SO is M 2 C 6 meq of Fe SO is contained in 3 millimo les of ferric sulphate

Physical Chemistry
GeneralBoard Competitive Exams 18 If the weight of metal chloride is x gram containing y gram of metal the equivalent weight of metal will be 1 E x35 5 3 E y x y x35 5 2 E 4 E 8 y x 8 x y y