Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
EnergeticsQ 14 b 300 R C 400 R Two moles of an ideal 500 R gas C R 3 52 against constant pressure of 2 atm which was initially at 350 K and 1 atm pressure The work involve in the process is equal to a 250 R was compressed adiabatically

Physical Chemistry
General80 kJ of heat is given to 36 g of water Then the a b 80 number of H and OH ions are 1 2044 10 4 each 80 number of water molecules that remain in solution is 36 18x6 02 x 1023x80 H 80 36 6 02 x 10 OH numbers of H and OH ions are 1 2044 x 10 24 80 c the ratio of H and OH d each ions

Physical Chemistry
GeneralA bowman is shooting arrows at a target Which of the following demonstrates high accuracy but low precision The bowman consistently hits to the left of the target The bowman consistently hits to the right of the bullseye 1 poin The bowman consistently hits around the target but never hits the bullseye The bowman consistently hits the bullseye The bowman consistently misses the target and hits a tree in the same spot
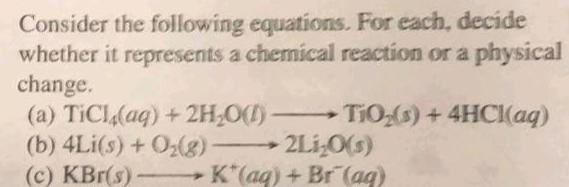
Physical Chemistry
GeneralConsider the following equations For each decide whether it represents a chemical reaction or a physical change TiO s 4HCl aq a TiCl aq 2H O b 4Li s O g c KBr s 2Li O s K aq Br aq

Physical Chemistry
General20 The values of four weak acids W X Y and Z are listed below Which acid is weakest Explain W X Y Z 21 What is the Br nsted Lowry conjugate base of Write an equation to illustrate your answer 22 Three aqueous solutions of nitric acid are listed below W X Y What is the order of increasing pH of these solutions 23 Four aqueous solutions are listed below W X

Physical Chemistry
Solid stateExample 1 9 In a CPS close packed structure of mixed oxides it is found that lattice has 02 oxide ions and one half of octahedral voids are occupied by trivalent cations A and one eighth of tetrahedral voids are occupied by divalent cations B2 Derive formula of the mixed oxide 3

Physical Chemistry
GeneralWhich of the solvent given in figure could be used to discriminate the acidities of PH3 pka 27 and GeH4 pKa 25 Fluorosulfuric acid H SO F Hydrofluoric acid HF 20 Sulfuric acid H SO Methanoic acid HCOOH 10 Ethanoic acid CH COOH Ethanol CH CH OH Water H O Dimethylsulfoxide DMSO 2 points Ammonia NH 20 30 0 10 Effective pH in water 40

Physical Chemistry
EnergeticsIf AH is the change in enthalpy and AE the change in internal energy accompanying a gaseou reaction then 1 AH is always greater than AE 2 AH AE only if the number of moles of the products is greater than the number of moles of the reactants 3 AH is always less than AE 4 AH AE only if the number of moles of products is less than the number of moles of the reactants

Physical Chemistry
Energetics20 Water is brought to boil under a pressure of 1 0 atm When an electric current of 0 50 A from a 12 V supply is passed for 300 s through a resistance in thermal contact with it it is found that 0 798 g of water is vaporised Calculate the molar internal energy change at boiling point 373 15 K a 37 5 kJ mol c 42 6 kJ mol b 3 75 kJ mol d 4 26 kJ mol 1

Physical Chemistry
Equilibrium6 pH of a solution produced when an aqueous solution of pH 6 is mixed with an equal volume of an aqueous KCET 2012 solution of pH 3 is about a 3 3 b 4 3 c 4 0 pH of a saturated solution of Ba OH d 4 5 is 12 TI

Physical Chemistry
Energetics1 Regarding the internal energy of the molecule which of the following statement is correct 1 Its absolute value can be successfully calculated 2 Its absolute value cannot be determined 3 It is the sum of vibrational and rotational energies 4 Both 1 3

Physical Chemistry
GeneralFour elements arbitrarily labelled A 1 point B C and D have electronegativities 3 8 3 3 2 8 and 1 3 respectively Place the compounds AB AD BD and AC in order of increasing covalent character

Physical Chemistry
EquilibriumFor a weak acid the incorrect statement is Manipal 2 a its dissociation constant is low b its pKa is very low c it is partially dissociated d solution of its sodium salt is alkaline in water

Physical Chemistry
Nuclear chemistrycop In photoelectric effect if a weak intensity radiation instead of strong intensity of suitable frequency is used then 1 Photoelectric effect will get delayed 2 Photoelectric effect will not take place 3 Maximum kinetic energy will decrease 4 Saturation current will decrease

Physical Chemistry
EquilibriumQ 20 2 NOBr g 2 NO g Br g If nitrosyl bromide NOBr is 33 33 dissociated at 25 C a total pressure of 0 28 atm Calculate K for the dissociation at this temperature

Physical Chemistry
ElectrochemistryElectrochemical series helps us to calculate the standard EMF of any cell However if the con centrations of the electrolytes in the halfcells are not 1 M and temprature is not 298 K Nernst equation is used to know the exact EMf of the cell Further using the identical electrodes but with different concentrations of the elctrolyte in the two half cells a cell can be set up called concen tration cell For cell reactions in equilibrium Nernst equation can be applied to find the equilibrium constant of these reactions from the standard EMF of the cell 0 75 V E Fe Given Er Cr 3 will be A 0 2409V 3 0 45 V the EMF of the cell Cr Cr 0 1M Fe 0 01M Fe DY 0 2606 V B 0 3394 V C 0 30 V The Equlibrium constant of the cell reaction will be of the order of A 1010 B 1020 C 1030 D 1040 The iron electrodes are used in both the half cells but in one halfcell 0 1M FeSO4 solution is taken but in the other 0 01M FeSO solution is taken the EMF of the cell will be A 0 591 V B 0 0295 V C 0 0098 V D 0 0197 V 11

Physical Chemistry
Solid state29 A crystal is made of particle x y and z x forms f c c packing y occupies all the octahedral voides of x and z occupies all the tetrahedral voides of x If all particle along one body diagonal are removed the formula of the crystal would be 1 XYZ 2 3 X8Y4Z5 2 X YZ 4 X5Y4Z8

Physical Chemistry
General4 All triangular units 3 All tetrahedral units A 0 01 M ammonia solution is 5 ionized its pH will be 1 11 80 2 10 69 3 7 22 4 12 24 Which one of the following compounds is different from the rest in terms of undergoin hydrolysis to form simpler compounds 1 Sucrose 2 Maltose 3 Lactose 4 Glucose

Physical Chemistry
Gaseous and liquid statesNH OH with 25 15 cm mol at the same temperature are 1 275 6 0 91 2 275 6 9 1 3 266 6 9 6 4 30 84 At STP 0 48 g of O diffused through a porous partition in 1200 seconds What volume of will diffuse in the same time and under the same conditions 1 286 5 mL 2 346 7 mL Which of the following compounds is most acidic 3 112 2 mL 4 224 8 mL

Physical Chemistry
EquilibriumIn which of the following aqueous solution the degree of dissociation of water is maximum 1 NH4Cl solution 1 204 2 CH3COONa solution 3 CH3COONH solution 4 NaCl solution 1 201 5 The solubility of BaSO4 in water is 0 00233 g per litre at 30 C The solubility of BaSO4 in 0 1 M NH4 2SO4 colution at the same temperature is Ba 137

Physical Chemistry
GeneralCHEMISTRY Match the compound with the metal for which it is used for the process of extraction i NaCN ii lodine iii Cryolite a Titanium b Aluminium c Silver ore 1 i c ii a iii b 3 1 a ii c iii b Salicylic acid is produc 2 i c ii b iii a 4 1 b ii a iii c when phonol in alcoholic KOH is treated with
Physical Chemistry
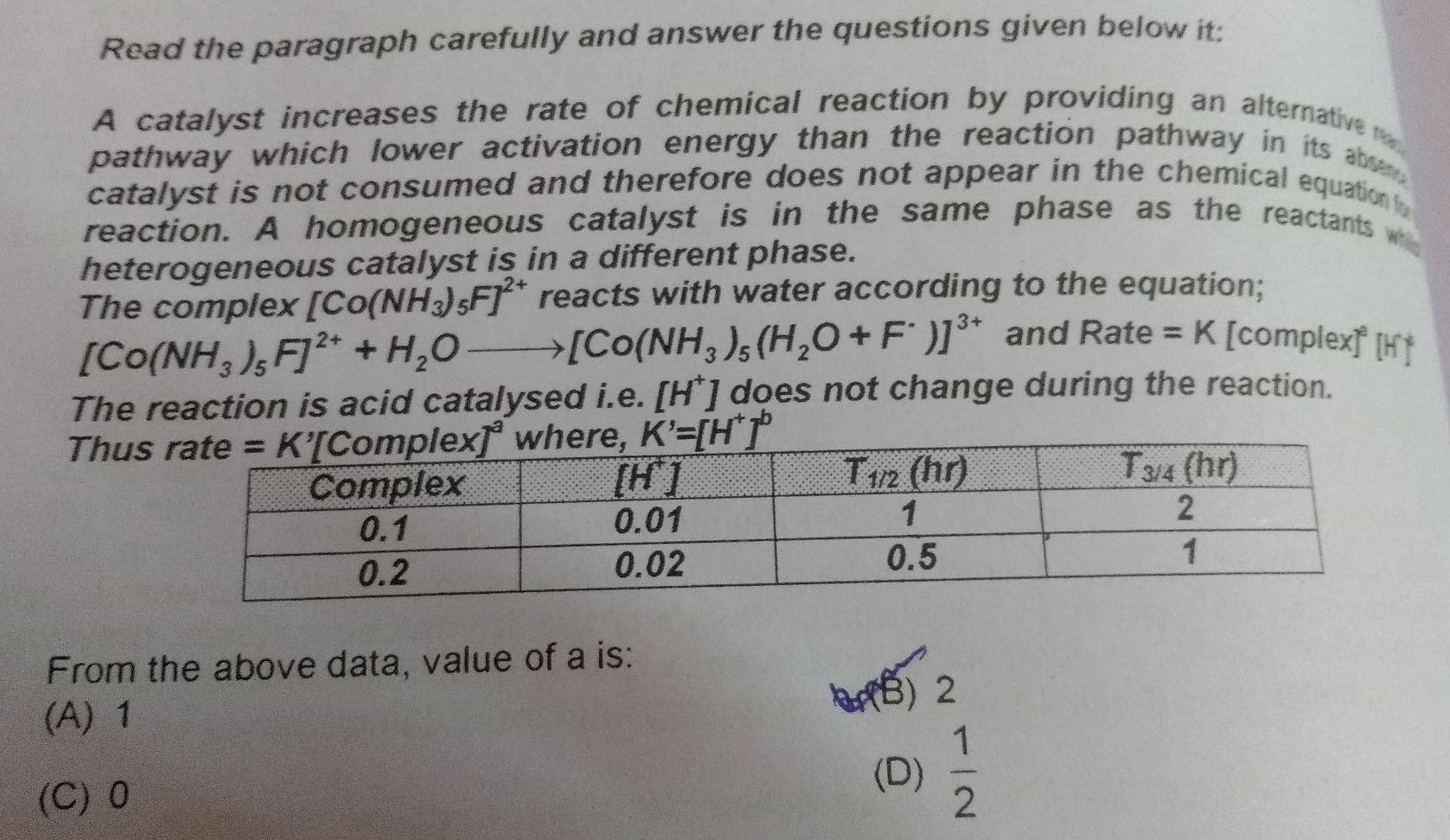
Chemical kineticsRead the paragraph carefully and answer the questions given below it TO A catalyst increases the rate of chemical reaction by providing an alternative pathway which lower activation energy than the reaction pathway in its absen catalyst is not consumed and therefore does not appear in the chemical equation fo reaction A homogeneous catalyst is in the same phase as the reactants heterogeneous catalyst is in a different phase The complex Co NH3 sF 2 reacts with water according to the equation Co NH3 H O F and Rate K complex H Co NH3 F 2 H O The reaction is acid catalysed i e H does not change during the reaction Thus rate K Complex where K H Complex 0 1 0 2 H 0 01 0 02 From the above data value of a is A 1 C O 3 T172 hr 1 0 5 BA 2 D NI 2 T3 4 hr 2 1

Physical Chemistry
Chemical kineticsPre Medical Chemistry 12 The rate of first order reaction is 1 5 x 10 2 mol L 1 min at 0 5 M concentration of the reactant The half life of the reaction is 1 7 53 min 3 23 1 min 2 0 383 min 4 8 73 min 13 In a first order reaction the concentration of the from 0 8 M to 0 4 M in

Physical Chemistry
General14 If 50 ml of M 50 NaOH 20ml of M 40 KOH and 30 ml of M 60 CSOH are mixed then OH concentration in resultant solution will be 1 2 10 2 M 3 2 0 M 2 1 x 10 M 4 5 10 2 M

Physical Chemistry
EquilibriumWhat is the correct dissociation constant expression for the following reaction HCHO aq H O 1 H3O aq CHO aq O Kb O Ka HO CHO RCH K Oks HCHO H CHO HOCHO HCHO HCHO B9 H

Physical Chemistry
Electrochemistry25 What conclusion about the unknown substance can be inferred from the graph below Solubility of Unknown Substance in Water at 1 atm A The substance is a supersaturated sa salt B The substance is an unsaturated salt C The substance is a gas

Physical Chemistry
Atomic StructureThe radial probability function which provides us the probability of finding an electron in a spherical shell of thickness dr at a distance of r from the nucleus for the hydrogen atom is plotted here for an orbital having a value of O for I The orbital is 10 40 30 20 10 2S 2Pz 3d O 1 0 1 point 200 400 600 800 10001200 1400

Physical Chemistry
SolutionsId The rate constant for the second order reaction 2NO g 2NO g O g is 0 54 M s at 300 C How long in seconds would it take for the concentration of NO to de 0 62 M to 0 28 M 3 pts

Physical Chemistry
Chemical BondingAssertion Nitrogen and oxygen are the main components in the atmosphere but these do not react to form oxides of nitrogen JEE Main 2015 Reason The reaction between nitrogen and oxygen requires high temperature A Both the assertion and reason are incorrect B Both assertion and reason are correct and the reason is the correct explanation for the assertion C Both assertion and reason are correct but the reason is not the correct explanation for the assertion D The assertion is incorrect but the reason is correct

Physical Chemistry
GeneralA AgNO3 solution containing 0 00739 g of AgNO3 per gram of water is electrolyzed between Ag electrodes During the experiment 0 078 g silver was deposited on the cathode At the end of the experiment anode solution contains 23 14 g of silver nitrate What is the transport number of silver ion Atomic weight of silver and molecular weight silver nitrate are 108 and 170 respectively 3 3

Physical Chemistry
General11 Phosphoric acid H3PO4 is a weak electrolyte because it A forms a number of different ions B dissociates incompletely in solution C consists of more than two atoms rotons to solution

Physical Chemistry
EnergeticsIn the balanced redox reaction x Cu O y NO3 14 H 6Cu NO 7H O the n facto of Cu O and NO3 is 2 and 3 respectively Statement 2 Since reciprocal of n factor s ratio is molar ratio and so x y 2 3 A Statement 1 is true Statement 2 is true and Statement 2 is correct explanation for Statement 1 B Statement 1 is true Statement 2 is true and Statement 2 is NOT correct explanation for Statement 1 C Statement 1 is true Statement 2 is false D Statement 1 is false Statement 2 is true Statement 1

Physical Chemistry
Gaseous and liquid states112 302 CH4 kept in a container A hole was made in the container and gases are allowed to escape After 3 hours the order of partial pressure in the container is Options Pso PcH4 PH PH Pso PCH4 PCH Pso PH

Physical Chemistry
Atomic StructureChoose the correct statement among the following Radial distribution function y 4r dr give probability at a particular distance along one chosen direction p r give probability density at a particular distance over a spherical surface 1 11 III IV A II IV For s orbitals P r Y 0 T T x y z is independent of 9 and 2p orbital with quantum numbers n 2 2 1 m 0 also shows angular dependence B II III IV C I III IV D III N

Physical Chemistry
Energetics9 A system absorbs 10 kJ of heat and does 4 kJ of work The internal energy of the system 1 Decreases by 6 kJ 2 Increases by 6 kJ 3 Decreases by 14 kJ

Physical Chemistry
Energetics24 Which compound will absorb the maximum amount of heat when dissolved in the same amount of water Integral heats of solution at 25 C in kcal mol of each solute are given in brackets 1 HCI AH 17 74 2 HNO AH 7 85 3 NH NO NH 16 08 4 NaCl AH 1 02

Physical Chemistry
GeneralTwo solutions of H SO4 are prepared with different composition Solution A 500 mL of 1 M aq H SO4 having density 1 2 g mL Solution B 100 mL of 2 M aq H SO4 having density 1 4 g mL Select the correct statement If both solutions A and B are mixed then molarity of final solution is 2 33 M If both solutions A and B are mixed then w w of HOSO is 10 72 in final solution

Physical Chemistry
General8 Which property of water makes it well suited for the transport of gases and electrolytes through the human body F Its low molecular weight makes it an easy substance to pump G Its high polarity makes it an excellent solvent of many substances H Its high specific heat makes it resistant to temperature change 3 Its relative chemical stability makes it an excellent energy source

Physical Chemistry
Energetics3 18 69 kJ 15 If the heat of Na Nag O 2NO O Oza 1 2x z y 3 2x z y 4 1 13 kJ formation of NO is NO l the heat of reaction 2NO is y and the heat of reaction 2NO isz then 2 2y z x 4 2z x y

Physical Chemistry
Solutions48 If the osmotic pressure of a 0 010 M aqueous solution of sucrose at 27 C is 0 25 atm then the osmotic pressure of a 0 010 M aqueous solution of NaCl at 27 C is 3 0 25 atm 1 0 062 atm 2 0 12 atm 4 0 50 atm

Physical Chemistry
Solutions4 Acetone chloroform 490 Benzene and toluene form nearly ideal solutions At 20 C the vapour pressure of benzene is 75 torr and that of toluene is 22 torr The partial pressure of benzene at 20 C for a solution containing 78 g of benzene and 46 g of toluene in torr is 1 25 3 53 5 2 50 4 37 5 ans

Physical Chemistry
Gaseous and liquid statesArrange the following in correct order of Lewis acidity BF BCI BBr 3 BF BCI BBr 1 BF BBr BCI 2 BF BCI BBr Consider the following structures 4 BBr BF BCI

Physical Chemistry
Surface chemistryWhich of the following will have highest coagulating power for As S3 colloid a Po c A1 b so d Na

Physical Chemistry
Gaseous and liquid statesQuestion No 80 Which of the following is incorrect Options A real gas behaves like an ideal gas over a wide range of pressure at Boyle temperature An ideal gas can never be liquefied Below inversion temperature the gas can be heated by adiabatic expansion against vaccuum Inversion temperature is twice of Boyle s temperature

Physical Chemistry
General26 An athlete takes 100 g of glucose of energy equivalent to 1560 kJ How much amount of energy is uptaken by 1 g molecule of glucose 2 2808 kJ 4 28 08 kJ 1 15 6 kJ 3 1560 kJ

Physical Chemistry
Gaseous and liquid statesHelium atom is two times heavier than a hydrogen molecule At 298 K the average kinetic energy of a helium atom is Options Two times that of a hydrogen molecule Same as that of a hydrogen molecule Four times that of a hydrogen molecule

Physical Chemistry
Chemical kinetics72 An ideal solution contains two volatile liquids A P 100 torr and B P 200 torr If the mixture contains 1 mole of A and 4 moles of B then total vapour pressure of the distillate is 1 150 torr 3 188 88 torr 4 198 88 torr 2 180 torr
Physical Chemistry
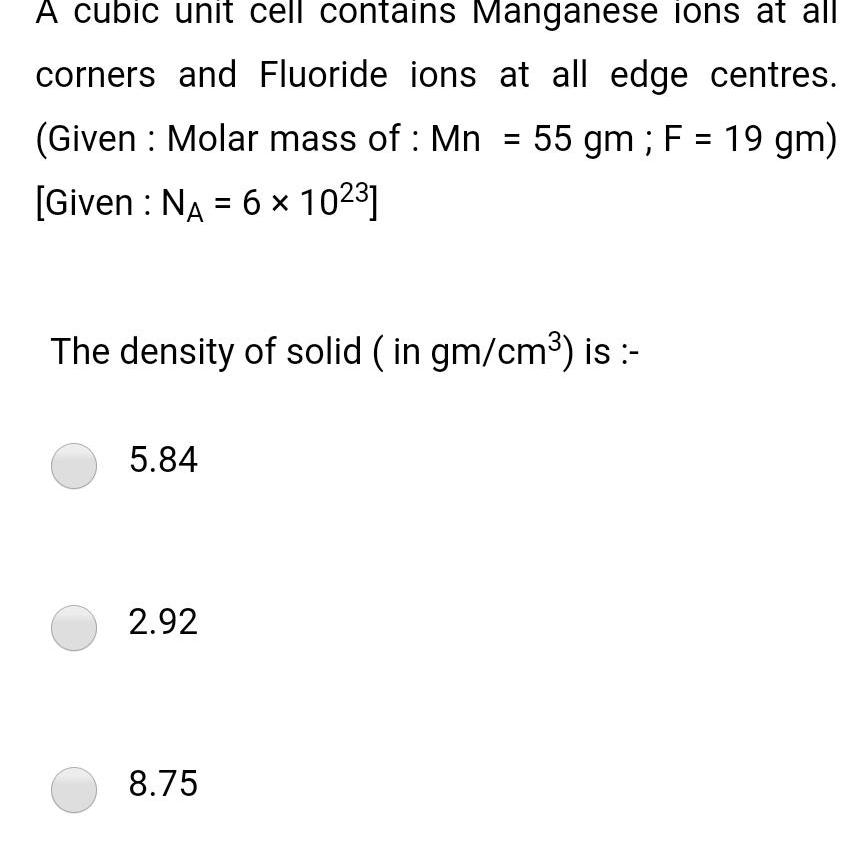
Solid stateA cubic unit cell contains Manganese ions at all corners and Fluoride ions at all edge centres Given Molar mass of Mn 55 gm F 19 gm Given NA 6 1023 The density of solid in gm cm is 5 84 2 92 8 75

Physical Chemistry
Chemical Bonding9 Given electronic configurations of four elements E E E3 and E4 are respectively 1s 1s 2s 2p 1s 2s 2p5 and 1s 2s 2p6 The element which is capable of forming ionic as well as covalent bonds is 1 E 2 E 3 E 4 E4

Physical Chemistry
EnergeticsThermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equation X 2 g 2X g The standard reaction Gibbs energy A G of this reaction is positive At the start of the reaction there is one mole of X2 and no X As the reaction proceeds the number of moles of X formed is given by B Thus Bequilibrium is the number of moles of X formed at equilibrium The reaction is carried out at a constant total pressure of 2 bar Consider the gases to behave ideally Given R 0 083 L bar K mol The equilibrium constant Kp for this reaction at 298 K in terms of Bequilibrium is A 8p equilibrium 2 Bequilibrium B 88 quilibrium 4 equilibrium C equilibrium 48 2 Bequilibrium JEE Advanced 2016 3 124 40 D equilibrium 4 Bequilibrium JEE Advanced 2016 3 124 The INCORRECT statement among the following for this reaction is A Decrease in the total pressure will result in formation of more moles of gaseous X B At the start of the reaction dissociation of gaseous X2 takes place spontaneously C Bequilibrium 0 7