Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Atomic StructureThe valence shell electronic configuration transition elements is 151 1 ns 2 ns np5 3 ns0 2 n 1 d1 10 4 ns n 1 10

Physical Chemistry
General500 mL of a glucose solution contains 6 02 x 1022 molecules The concentration of the solution 1 54 www com

Physical Chemistry
EquilibriumVE 5 What indicatro should be used for the titration of 0 10M KH BO with 1 10 MHCI

Physical Chemistry
Generaleryllium occurs naturally in the form of beryl The metal is produced from its ore lectrolysis after the ore has been converted to the oxide and then to the chloride How m rams of Be s is deposited from a BeCl solution by a current of 5 0 A that flows for 1 Atomic weight Be 9 a 0 840 b 1 68 c 1 42 d 1 08

Physical Chemistry
EquilibriumVapour density of PCI is 104 16 but when heated to 230 C its vapour density is reduced to 62 The degree of dissociation of PCI at this temperature will be 1 6 8 3 46 2 68 4 64 10225

Physical Chemistry
GeneralBy X ray diffraction it is found that nickel at mass 59 g mol crystallizes with ccp The edge length of the unit cell is 3 5 If density of Ni crystal is 9 0 g cm Then value of Avogadro s number from the data is a 6 05 x 1023 b 6 11 x 1023 c 6 02 x 1023 d 6 023 x 1023

Physical Chemistry
Atomic Structurea The schrodinger wave equation for hydrogen atom is 1 2 To r 2ag ao Wherea is Bohr s radius Let the radial node in 2s be at r Then find r in terms of a 4 2s 1 1 2 2 ao 2

Physical Chemistry
GeneralA B 2014 An aqueous solution of X is added slowly to an aqueous solution of Y as shown in List 1 The variation in conductivity of these reactions in List II Match List I with List II and select the correct answer using the code given below the lists List 1 S Codes C P C H5 3 N CH COOH X Y Q R KI 0 1M AgNO 0 01M X CH COOH KOH X NaOH HI X P 3 4 2 1 4 3 3 4 R2243 STTT 2 S 1 1 1 List II 1 Conductivity decreases and then increases 2 3 4 Conductivity decreases and then does not change much Conductivity increases and then does not change much Conductivity does not change much and then increases 2013

Physical Chemistry
Electrochemistry4 The electric charge required for electrode deposition of one gram equivalent of a substnace is b 96500 coulombs per second d charge on one mole of electrons a one ampere per second c one ampere for one hour 1

Physical Chemistry
EquilibriumEquations A K Q B AGO RT In Q ii Equilibrium C K Q iii Spontaneous 869 6 Type of processes i Non spontaneous D T AH ve iv Spontaneous 869 6 AS a A i B ii C iii D iv b A iii B iv C ii D i A iv B i C ii D iii d A ii B i C iv D iii and endothermic T more mey

Physical Chemistry
SolutionsThe indicator phenol red is half in the ionic form when pH is 7 2 If the ratio of the undissociated form to the ionic form is 1 5 find the pH of the solution With the same pH for solution if indicator is altered such that the ratio of undissociated form to dissociated form becomes 1 4 find the pH when 50 of the new indicator is in ionic form

Physical Chemistry
Electrochemistry3 ber of Faradays F required to produce 20 g of calcium from molten CaCl Atomic mass of Ca 40 g mol 1 is CaCl 3 z t 9600 1 2 3 4 4 1 2 N 2 N 2 40 M1 20
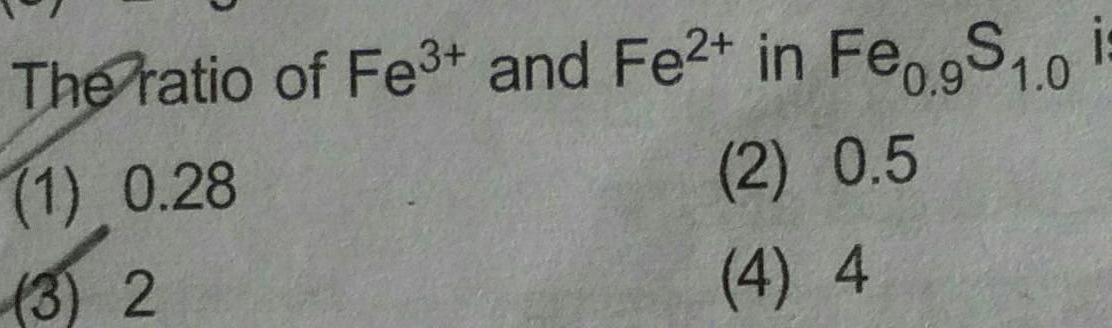

Physical Chemistry
Atomic StructureThe value of spin only magnetic moment for one of the following configuration is 2 84 B M The correct one is a d in strong field ligand b d in weak field ligand c d in weak as well as in strong field ligand d d in strong field ligand

Physical Chemistry
Chemical Bonding8 What is the mole fraction of the solute in a 1 00 m aqueous solution CBSE AIPMT 2015 a 0 177 b 1 770 c 0 0354 d 0 0177

Physical Chemistry
Gaseous and liquid states50 A closed bulb contains 0 01 mole of an inert gas He and NH4Cl s The initial pressure inside the bulb of helium at 27 C was 114 mm of Hg The bulb is now heated to 327 C when all the NH4Cl was decomposed and the final pressure becomes 908 mm of Hg Assuming ideal gas behaviour calculate a Partial pressure of HCl in mixture Amount of NH4Cl taken initially b
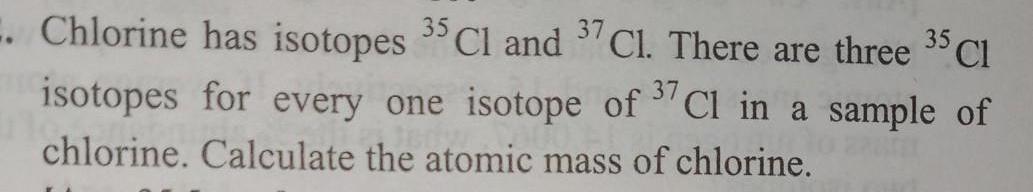
Physical Chemistry
Atomic StructureChlorine has isotopes 35 Cl and 37 Cl There are three 35 Cl isotopes for every one isotope of Cl in a sample of chlorine Calculate the atomic mass of chlorine 37

Physical Chemistry
General35 For every one 37Cl isotope there are three 35C isotopes in a sample of chlorine What will be the average atomic mass of chlorine a 35 b 37 c 35 5 d 35 6

Physical Chemistry
GeneralThe bonding in ammonium chloride a is covalent only b is electrovalent only c consists of three covalent nitrogen hydrogen bonds and an electrovalent bond between the ammonia molecules and the chlorine atom d consist of four covalent nitrogen hydrogen bonds and one electrovalent bond between the ammonium ion and chlorine atom

Physical Chemistry
Chemical kineticsdP 11 The reaction 2NO g H g N O H O follows the rate law N O K PNG PH dt If the reaction is initiated with PNo 1000 mm Hg and PH 10 mm Hg then the reaction will foll NO 1 Third order kinetics 2 Second order kinetics 3 First order kinetics 4 Zero order kinetics

Physical Chemistry
EnergeticsA schematic plot of In Keq versus inverse of temperature for a reaction is shown below 6 0 In Kea 2 0 1 5 10 T The reaction must be 1 exothermic 2 endothermic K 2 0 10 3 3 one with negligible enthalpy change A highly spontaneous at ordinary temperature M

Physical Chemistry
Solid stateestiloss to songor ai er Silver Atomic weight 108 g mol has a density of 10 5 g cm The number of silver atoms on a surface of area 10 2 m can be expressed in scientific notation as y 10 The value of x is de Mil Vot A 2 B 3 D 7 adh gnieu beau C 5 Made atan 106 10 to 6 29 definito

Physical Chemistry
Solid state70 How many nearest and next nearest neighbours respectively does potassium have in bcc lattice a 8 8 educ c 6 8 T 71 b 8 6 ng of d 6 6

Physical Chemistry
General267 A 20 solution of KOH density 1 02g ml has molarity 3 64 A 1 33 B 3 64 C 3 31 D 4 41

Physical Chemistry
Solid stateIn a H like atom the electron is excited to 5th energy level The total line in Paschen series and Balmer series are If it comes down by maximum transition 1 2 3 3 2 1 4 0

Physical Chemistry
Chemical kinetics12 Which of the following statements is incorrect a A second order reaction must be a bimolecular elementary reaction b A bimolecular elementary reaction must be a second order reaction c Zero order reaction must be a complex reaction d First order reaction may be complex or elementary reaction

Physical Chemistry
Atomic StructureWhat is the maximum number of orbitals that can be identified with the following quantum numbers n 3 1 1 m 0 AIPMT 2014 1 01 Bel 2 2 4 4 1 1 3p orbital 3 3 11 41 LL L m

Physical Chemistry
GeneralThe temperature rises by 18 F What is the rise on the Celsius scale ns 10 C

Physical Chemistry
Gaseous and liquid statesTwo flasks A B of equal capacity of volume contain NH and SO gas respectively under similar conditions Which flask has more number of moles 1 A 2 B 3 Both have same moles

Physical Chemistry
Equilibrium7 CE0138 In a reaction equilibrium proceeds towards reactants then K will be 1 K 1 3 K 0 2 K 1 4 K 1 CE013

Physical Chemistry
EquilibriumA buffer of pH 9 26 is made by dissolving x moles of ammonium sulphate and 0 1 mole of ammonia into 100 mL solution If pK of ammonia is 4 74 calculate value of x b NH4h Sou s2Nhat so

Physical Chemistry
GeneralBasic strength of NH OH in presence of NH C 1 Increases 2 Remains unchanged 3 Decreases 4 Some times increases or sometimes decreases

Physical Chemistry
ElectrochemistryDegree of dissociation of 0 1 N CH COOH is constant 1 x 10 5 2 10 4 3 10 4 10 2 Dissociation 1 10 5

Physical Chemistry
GeneralAmong the following processes identify those in which th change in internal energy AU is zero i isotherma compression of an ideal gas ii adiabatic expansion of a ideal gas iii free adiabatic expansion of an ideal go iv reversible cyclic process v irreversible cyclic process

Physical Chemistry
Chemical kineticsA radioactive element gets spilled over the floor of a room Its half life period is 30 days If the initial activity is ten times the permissible value after how many days will it be safe to enter the room 1 1000 days 3 10 days 2 300 days 4 100 days MAINS 2007

Physical Chemistry
Equilibrium21C1 g 12 g Cl2 g Equilibrium constant K for given reaction is 0 14 If initally 0 6M ICI was taken then what will equilibrium concentration of I is 1 0 1275 M 2 0 3250 M 3 0 35 M 4 0 2550 M

Physical Chemistry
Gaseous and liquid statesVapour pressure of one molal aqueous solution of AgNO3 at the normal boiling point of water assuming 100 ionisation is 1 787 05 torr 2 733 56 torr 3 720 12 torr 4 700 torr pole in le no

Physical Chemistry
GeneralA sample was weighted using two different balances The results were i 3 929g ii 4 0 g How would the weight of the sample be reported a 3 93g c 3 9g b 3g d 3 929g

Physical Chemistry
Solutionssolut mole fraction of M the following figure Here XL and XM represent mole fractions of L and M respectively in the solution The correct statement s applicable to this system is are c PL 1 Z 0 the deviath XM A Attractive intermolecular interactions between L L in pure liquid L and M M in pure liquid M are stronger than those between L M when mixed in solution B The point Z represents vapour pressure of pure liquid M and Raoult s law is obeyed when XL O C The point Z represents vapour pressure of pure liquid L and Raoult s law is obeyed when XL 1 D The point Z represents vapour pressure of pure liquid M and Raoult s law is obeyed from 2017 XL 0 to XL 1 019

Physical Chemistry
EnergeticsOne mole of ice is converted into water at 273 K The entropies of H O s and H O I are 38 20 and 60 01 J mole K respectively The enthalpy change for the conversion is a 59 54 J mol b 5954 J mole d 320 6 J mole c 594 5 J mole

Physical Chemistry
Generalat A 20 litre container at 400 K contains CO2 g a pressure 0 4 atm and an excess of SrO neglect the volume of solid SrO The volume of the container is now decreased by moving the movable piston fitted in the container The maximum volume of the container when pressure of CO2 attains its maximum value will be Given that SrCO3 s SrO s Kp 1 6atm 1 10 litre 3 2 litre 2 4 litre 4 5 litre CO g

Physical Chemistry
GeneralAt 518 C the rate of decomposition of a sample of gaseous acetaldehyde initially at a pressure 363 Torr was 1 00 Torr s when 5 had reacted and 0 5 Torr s when 33 had reacted The order of the reaction is 1 O 3 3 2 2 4 1 MAINS 2018

Physical Chemistry
GeneralFor the reaction PC13 g Cl g of equilibrium can be shifted to the right by a Increasing the temperature b Doubling the volume PCI g the position c Addition of Cl at constant volume d Addition of equimolar quantities of PCI and PC1

Physical Chemistry
Gaseous and liquid statesB 8 Calculate the radius of He atoms if its Vander Waal s constant b is 24 ml mol 1 cubic centimeter Note 1 ml A 1 355 A B 1 314 A C 1 255 A D 0 355 A

Physical Chemistry
Chemical Bondingelectrons In which of the following pairs the radii of second species is greater than that of first 1 K Ca 2 H He 3 Mg Mg2 4 0 0

Physical Chemistry
EnergeticsGiven that the temperature coefficient for the saponification of ethyl acetate by NaOH is 1 75 Calcu activation energy for the saponification of ethyl acetate Let initial temperature is 298K Use In 1 75 0 561

Physical Chemistry
SolutionsGold number of gum arabic is 0 15 The amount of gum arabic required to protect 100 ml of gold sol from coagulation by 10 ml of 10 NaCl solution is A 0 15 millimoles B 0 15 mg C 1 5 millimoles D 1 5 mg

Physical Chemistry
General4 A 200 g sample of hard water is passed through the column of cation exchange resin in which H is exchanged by Ca The outlet water of column required 50 mL of 0 1M NaOH for complete neutralization What is the hardness of Ca ion in ppm A 250 ppm B 500 ppmC 750 ppmD 1000 ppm

Physical Chemistry
Electrochemistryred contained 0 1M MnO MnO 8H 5e Calculate the potential of an indicator electrode versus the standard hydrogen electrode which originally and 0 8M H and which was treated with Fe2 to reduce 90 of the MnO to Mn Mn H O E 1 51V Cal

Physical Chemistry
Nuclear chemistryThe half life period of a first order reaction is 10 minutes The time required for the concentration of the reactant to change from 0 08 M to 0 02 M is B 20 min D 40 min A 10 min C 30 min