Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
GeneralFor a reversible reaction 2NO g O g 2NO g the rate expression is given as 2 6x10 NO 0 4 1 NO The rate constant of forward reaction will be dt net dx A 1 58 10 3 X C 10 66 10 B 2 6 10 3 D 1 06 10

Physical Chemistry
EquilibriumAt a certain temperature solids NH HS and NH COONH are introduced into an evacuated vessel Consider the dissociation equilibrium of the species NH COONH s 2NH g CO 9 Kp Kp NH HS s NH g H S They are allowed to simultaneously come to equilibrium Select the correct statement 1 The ratio of amount of NH3 produced by the species is equal to the ratio of their K 2 Total pressure exerted by NH3 is greater than the total pressure exerted by CO and H S 3 Mole fraction of NH3 cannot be greater than 1 3 4 Pressure exerted by CO is twice the pressure overted by

Physical Chemistry
Solid stateThe most unsymmetrical and symmetrical systems are respectively a Tetragonal Cubic b Triclinic Cubic c Rhombohedral Hexagonal d Orthorhombic Cubic The crystal system of a compound with unit cell paror

Physical Chemistry
EnergeticsCalculate the heat of formation of methane in kcal mol using the following thermo chemical reactions C s O g CO g AH 94 2 kcal mol H g O g H O l AH 68 3 kcal mol CH g 202 g CO g 2H O l AH 210 8 kcal mol a 45 9 c 20 0 b 47 8 d 47 3


Physical Chemistry
Energeticsvi The equivalent mass of KMnO4 is x what is the insufficiency in this statement

Physical Chemistry
Gaseous and liquid statesIn a 13 L vessel initially following reaction occur C s S g CS g by 12 g C 64 g S 76 g CS at 1027 C temperature then total pressure is 1 200R 2 158R 3 100R 4 79R

Physical Chemistry
Gaseous and liquid states3 Two gases P and Q having ratio of rate of diffusion is 1 2 and the ratio of their masses present in the mixture is 1 3 then the ratio of their mole fraction would be 1 2 5 3 5 9 2 1 6 1 3 4 1 12 1 3

Physical Chemistry
GeneralCalculate H of 0 01 M A B salt solution if K HB 10 2 a A 10 M B 10 7 M C 6 2 x 10 3 D 1 6 x 10 12 M PHA 11 12

Physical Chemistry
SolutionsEquimolar solutions of KCl and compound X in H O show depressions in freezing point in the ratio of 4 1 Assuming KCl to be completely ionized the compound X in solution must A Dissociate to the extent of 50 B Hydrolyse to the extent of 80 C Dimerize to the extent of 50 mi of D Trimerize to the extent of 75

Physical Chemistry
Generalii Suppose the human population of the world is 3 x 1010 If 100 molecules of sugar C12H22011 are distributed per head what is the total quantity of sugar required for distribution

Physical Chemistry
SolutionsAn element X of atomic mass 25 g exists as X4 in benzene to the extent of 100 When 10 30 g of saturated solution of X in benzene is added to 20 0 g of benzene the depression in freezing point of the resulting solution is 0 51 K IfK of benzene is 5 1 K kg mol the solubility of X in 100 g of benzene will be A 2 9 g B 3 0 gute eet brom C 0 7 g bD 0 3 g


Physical Chemistry
GeneralExample 17 Calcium phosphide Ca P formed by reacting calcium orthophosphate Ca3 PO4 2 with magnesium was hydrolysed by water The evolved phosphine PH3 was burnt in air to yield phosphorus pentoxide P O5 How many grams of magnesium metaphosphate would be obtained if 19 2 g of magnesium were used for reducing calcium phosphate At wt Mg 24 P 31 Ca3 PO4 2 Mg Ca3P MgO Ca3P H O Ca OH PH PH O P O5 H O MgO P O5 Mg PO3 2 Magnesium metaphosphate
Physical Chemistry
GeneralA beaker containing 18 g of glucose in 100g H O and another containing 18 g of urea in 100g H O places under a bell jar and air is removed After a course of time when equillibrium reaches how much water will be transferred from one beaker to the other A 20g 0 B 25g8 2 C 30g 2 A D 50g

Physical Chemistry
Atomic StructureO Calculate wavelength of an electron moving with a velocity of 2 05 107 ms Mass of electron 9 1 10 31 kg

Physical Chemistry
GeneralThe volume of a gas in discharge tube is 1 12 x 107 mL at STP Then the number of molecule of gas in the tube is 1 3 01 x 104 3 3 01 x 10 2 2 3 01 x 1015 X 4 3 01 x 1016

Physical Chemistry
GeneralIn which of the following compounds a exhibits two different oxidation states b NH NO a NH OH d N H c N H at of the following arrang an elemen

Physical Chemistry
EquilibriumImpure copper containing Fe Au Ag as impurities is electrolytically refined A current of 140 A for 482 5 s decreased the mass of the anode by 22 26 g and increased the mass of cathode by 22 011 g Percentage of iron in impure copper is Given molar mass of Fe 55 5 g mol molar mass of Cu 63 54 g mol a 0 95 b 0 85 c 0 97 KCET 2014 d 0 90

Physical Chemistry
Atomic Structure1 22 10 eV Calculate the energy of photon in kcal mol while wave number of photon is 1 cm Wave Moun number 1cm 1 10 m 10 m 1
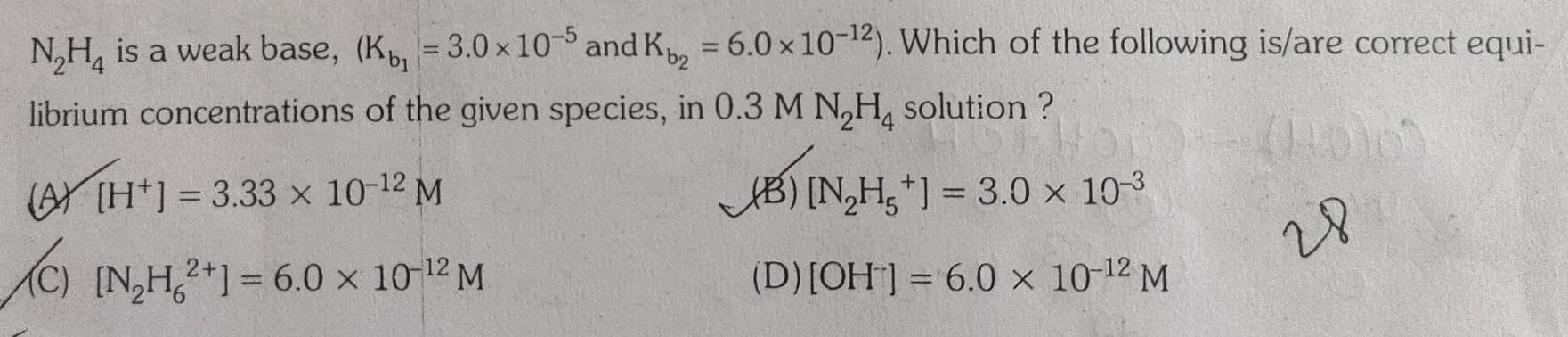
Physical Chemistry
EquilibriumN H is a weak base K 3 0 x 10 5 and K 6 0 x 10 2 Which of the following is are correct equi librium concentrations of the given species in 0 3 M N H solution 3 33 x 10 12 M B N H 3 0 10 3 C N H2 1 6 0 10 2 M D OH 6 0 x 10 2 M 28

Physical Chemistry
EquilibriumWhat is the pH of 4 x 10 3 MY OH solution assuming the first dissociation to be 100 and second dissociation to be 50 where Y represents a metal cation log 2 0 3010 log 3 0 477 A 11 778 B 11 477 C 2 523 D 2 222

Physical Chemistry
GeneralMole C A sample of ammonium phosphate NH4 3PO4 contains 3 18 moles of hydrogen atoms The number of moles of oxygen atoms in the sample is 55151 1 0 265 3 1 06 2 0 795 4 4 00

Physical Chemistry
EquilibriumInstance 8 0 24 mol of each SO3 g and NO g were taken in a reaction vessel and heated to some temperature where the following equilibrium was established SO3 g NO g SO g NO g Unreacted SO3 at equilibrium were absorbed in a 250 mL 1 5 M NaOH solution 25 mL of this solution required 9 3 mL of a 1 0 M HCl solution for complete neutralization Determine K

Physical Chemistry
SolutionsPartial pressure needed to dissolve 21 mg of CO in 100 g of H O at 298 K is K of CO is 2 937 K Pa m mol 1 14 0 kpa 2 7 0 kpa DE THE OPT 3 121 0 kpa S 4 79 0 kpa

Physical Chemistry
Solid state4 A compound has BCC unit cell with edge length 10 if density is 2 g cm then molar mass of the compou is 1 240 3 350 2 300 4 280

Physical Chemistry
GeneralThe electron gain enthalpy Heg of an element A is 200 kJ mol The electron affinity A of A a absolute temperature can be 200 kJ mol 2 201 kJ mol 4 100 kJ mol 3 200 kJ mol
Physical Chemistry
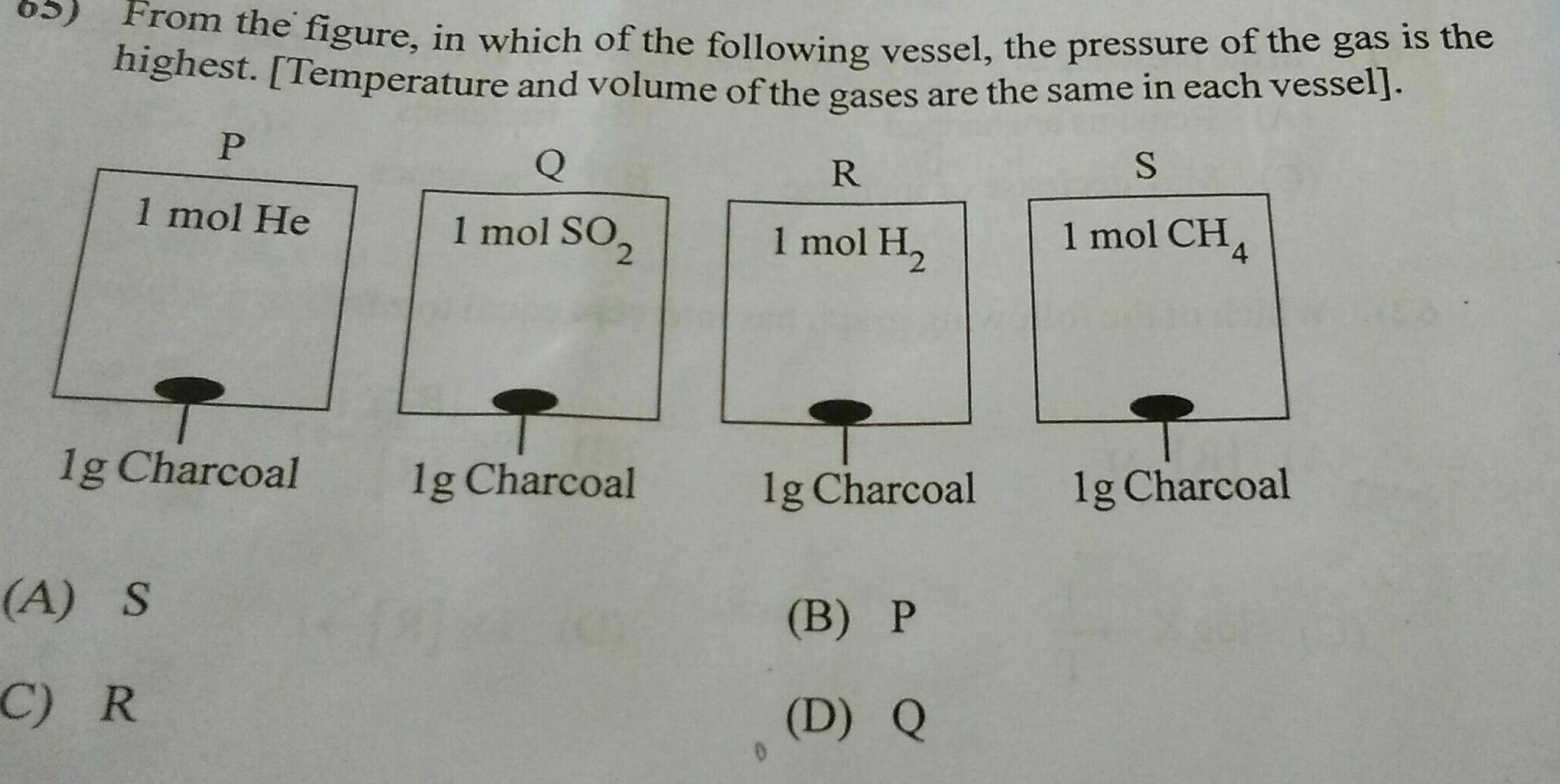
GeneralFrom the figure in which of the following vessel the pressure of the gas is the highest Temperature and volume of the gases are the same in each vessel S P 1 mol CH4 1 mol He lg Charcoal A S C R Q 1 mol SO 2 lg Charcoal R 1 mol H lg Charcoal B P D Q lg Charcoal

Physical Chemistry
General00 One litre of a mixture containing BaF and H SO4 was taken for analysis 25 mL of this mixture was treated with 20 0 mL of 0 1 NKOH for complete neutralisation Another 25 mL of the mixture was added to 100 mL of 0 05 N K CO3 solution and precipitate was filtered off The filtrate required 12 mL of 0 025 M oxalic acid solution using phenolphthalein as indicator Find the strength of BaF and H SO4 in mixture Ans BaF 6 372 g L H SO4 3 92 g L

Physical Chemistry
GeneralDecomposition of A g follows first order kinetics according to the reaction A g 2B g C g If initial pressure of A g is 10 atm and total pressure after 70 sec is 20 atm then In2 0 7 A Rate constant of reaction is 0 01 sec B Partial pressure of B at 70 sec is 10 atm C Partial pressure of C at 140 sec is 7 5 atm D Initial rate of reaction is 0 1 atm sec 13

Physical Chemistry
GeneralThe number of electrons in 1 6 g of CHA approximately 1 25 x 1024 3 6 1023 2 1 5 1024 4 3 0 1024

Physical Chemistry
Energeticsconstant of H S and HS are respectively 107 and 10 13 The pH of 0 1 M aqueous solution of H S will be 1 4 85 The dissociation 2 3 4 2 5 DI

Physical Chemistry
Solid stateTungsten has an atomic radius of 0 136 nm The density of tungsten is 19 4 g cm What is the crystal structure of tungsten Atomic weight W 184 a Simple cubic b Body centered cubic d None of these c Face centered cubic face centered cubic cell is 1 83 g cm at 20 C What is th

Physical Chemistry
Generalper second is xx 10 0 Find the value of x 6x10 305 0 x24x365 Rahul Dravid wants to wear 6 023 1021 Ag atoms in the form of a ring His Silver Gold Copper alloy ring consists of 20 of Silver The mass of the ring is 0 9x What is x both the sides

Physical Chemistry
ElectrochemistryIf three Faradays F of electricity is passed through the solutions of AgNO3 CuSO4 and AuCl3 the molar ratio of the cations deposited at the cathode is a 1 1 1 b 1 2 3 c 3 2 1 d 6 3 2

Physical Chemistry
Chemical kinetics3 In a reaction between A and B the initial rate of reaction To was measured for different initial concentrations of A and B as given below A mol L 1 0 20 B mol L 1 0 30 ro mol 0 20 0 40 0 10 0 05 L s 5 07 x 105 5 07 105 1 43 x 104 The order of the reaction with respect to A is a 1 5 b 0 5 c 1 d 2

Physical Chemistry
ElectrochemistryEthane gas is obtained in Koble s electrolysis of CH3COONa according to the following reaction CH3 2CH3COO 1 2CO 2e CH3 What volume of gas ethane gas at S T P would be obtained by a current of 0 5 amp 80 efficient if the current is passed for 965 min 1 1 8 L 2 2 688 L 4 11 2 L 3 5 45 L

Physical Chemistry
General25 ml of aqueous solution of BaCl required 20 mol of 1M Ag NO when titrated using K CrO indicator Assuming 100 ionisation the elevation of boiling paint BaC1 solution is K H O 0 52 K kg mol 1 0 21 K 2 0 42 K 3 0 62 K 974 0 72

Physical Chemistry
Gaseous and liquid statesA 4 56 Pure O diffuses through an aperture in 224 second whereas mixture of O and another gas containing 80 O diffuses from the same in 234 second The molecular mass of gas will be A 51 5 B 48 6 C 55 D 46 6 onde of 200 cm long tube HCI gas through inlet X and NH

Physical Chemistry
SolutionsThe vapour pressure of pure benzene at 50 C is 268 Torr How many moles of non volatile solute per mol of benzene is required to prepare a solution of benzene having a vapour pressure of 167 Torr at 50 C A 0 377 pe C 0 623 D 0 395 B 0 605 29705110CESIA

Physical Chemistry
GeneralWhich contains greater number of oxygen atoms 1 1 gm of O 2 1 gm of O 3 1 gm of 03 4 All have same number of oxygen atoms

Physical Chemistry
GeneralAn intimate mixture of ferric oxide Fe O3 and aluminium Al is used in solid fuel rockets Calculate the fuel value per gram and fuel value per cc of the mixture Heats of formation and densities are as follows H Al2O3 399 Kcal mol and Hr Fe O3 199 Kcal mol Density of Fe O3 5 2 g cc Density of Al 2 7 g cc 1988

Physical Chemistry
Atomic StructureJEE Advance 2019 Answer the following by appropriately matching the lists based on the information given in the paragraph Consider the Bohr s model of a one electron atom where the electron moves around the nucleus In the following List I contains some quantities for the nth orbit of the atom and List II contains options showing how they depend on n List I Radius of the nth orbit Angular momentum of the electron in the nth orbit Kinetic energy of the electron in the nth orbit Potential energy of the electron in the nth orbit 1 III IV List II P Q xn m n x n n xn 2 Which of the following options has the correct combination considering List I and List II A IV Q B III P C IV U D III S S T Which of the following options has the correct combination considering List I and List II A 1 T B II Q D II R C 1 P

Physical Chemistry
Chemical kinetics45 The activation energy for the reaction H O2 H O 0 1 2 is 18 K cal mol at 300 K calculate the fraction of molecules of reactonts having energy equa to or greater than threshold energy Anti log 13 02 9 36 x 10 14 1 9 36 x 10 14 2 1 2 x 10 12 3 4 2 x 10 16 4 5 2 x 10 15

Physical Chemistry
Chemical Bondinga 95 c 75 d 65 Al2 SO4 3 solution of 1 molal concentration is present in 1 litre solution of density 2 684 s cc How many moles of BaSO 4 would be precipitated on adding excess of BaCl2 in it b 3 moles a 2 moles c 6 moles 5 A certain public water supply contains 0 10 ppb part per billion of chloroform CHCI 3 How d 12 moles selecules of CHCl would be obtained in 0 478 ml drop of this water

Physical Chemistry
Generaland F are 72 pm and 136 pm respectively then choose 5 Atomic radius and ionic radius of F the CORRECT statement s A Ratio of size in term of volume 6 75 B increase in size in term of volume 5 75 in formation of F to F C Ratio of size in term of volume 675 du D increase in size in term of volume 575

Physical Chemistry
Chemical kineticsIn the reaction P QR S The time taken for 75 reaction of P is twice the time taken for 50 reaction of P The concentration of Q varies with reaction time as shown in the figure The overall order of the reaction is A 2 C 0 B 3 D 1 Qlo Q Time 201

Physical Chemistry
GeneralIn a compound Carbon is 52 2 Hydrogen is 13 Oxygen is 34 8 are present and molecular mass of the compound is 92 Calculate molecular formula of the compound

Physical Chemistry
Gaseous and liquid statesB 3 Calculate the volume occupied by 2 0 mole of N at 200 K and 8 21 atm pressure PcVc RTC 8 3 and 2 4 P V T

Physical Chemistry
Generaltion 1 If the mass of neutrons is doubled mass of electron is halved then find out the new atom of C12 and the percent by which it is increased Step 1 C 2 e 6 1 p 6 6 amu n 6 6 amu 12 amu If the mass of neutrons is doubled and mass of er is halved then n 12 amu 1 Increment 18 amu p 6 amu Note mass of er is negligible so it is not considered in atomic mass Final mass Initial mass Initial mass Step 2 100 18 12 12 100 50