Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
ElectrochemistryPure water is saturated with pure solid AgCl a silver electrode is placed in the solution and the potential is measured against normal calomel electrode at 25 C The experiment is then repeated with a saturated solution Agl If the difference in potential in the two cases is 0 177V What is the ratio of solubilities of AgCl and Agl at the temperature of the experiment a 10 c 10 b 106 d 104

Physical Chemistry
EquilibriumThe following equilibrium is established when hydogen chloride is dissolved in acetic acid HCI CH COOH CI CH COOH The set that characterizes the conjugate acid base pairs is A HC1 CH COOH and CH COOH Cl B HCI CH COOH and CH3COOH Cl C CH COOH HCI and CF CH COOH D HCl Cl and CH COOH CH COOH

Physical Chemistry
Atomic Structure23 Select the incorrect graph for velocity of e in an orbit vs Z n a V sad to zibst b 1 n 890 d c 1 sib dom at 107 bo z and n dro d a de ribet af mis avi n
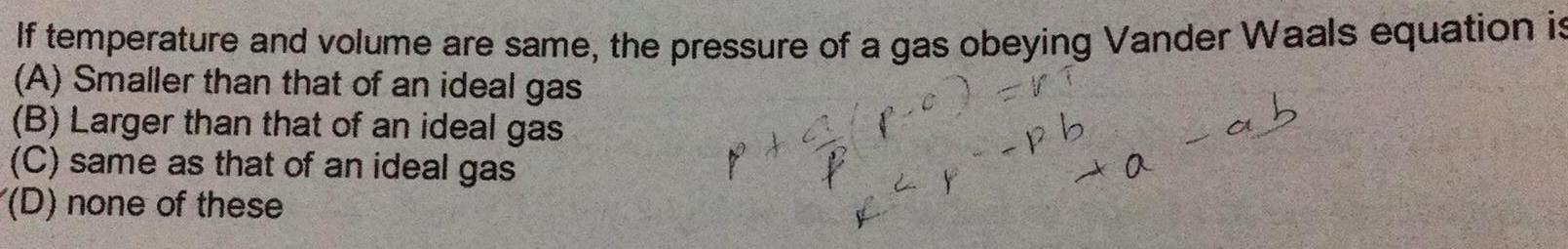
Physical Chemistry
Gaseous and liquid statesIf temperature and volume are same the pressure of a gas obeying Vander Waals equation is A Smaller than that of an ideal gas B Larger than that of an ideal gas P C ab C same as that of an ideal gas D none of these P 4 P pb a

Physical Chemistry
Chemical kinetics100 ml of liquid A and 25 ml of liquid B is mixed to give a solution which does not obey Raoult s law T volume of the solution 1 Will be 125 ml 3 Can be or than 125 ml 2 Can be or than 125 ml 4 Will be less than 125 ml pon 672 Kond pure nitric acid boils at 350 K TH

Physical Chemistry
General1 1 M 8 0 3 M If an ideal solution is made by mixing 2 moles of benzene p 266 mm Hg and 3 moles of another liquid 2 236 mm Hg The total vapour pressure of the solution at the same temperature would be 4 3 1 502 mm Hg 3 600 mm Hg 00 2 248 mm Hg 4 250 6 mm Hg ml of liquid A and 25 ml of liquid B is mixed to give a solution which does not obey Raoult s law The olution 21 Can be or than 125 ml

Physical Chemistry
Atomic StructureColumn I A No of orbitals in the n shell B Max no of electrons in a subshell C No of subshells in n shell Column II P 2 2 1 Q n R 2 1

Physical Chemistry
Generalg mixture of CaCO3 and NaCl reacts completely with 100 ml of HCI The of CaCO in the N 10 mixture is 1 40 500 2 60 4 80

Physical Chemistry
GeneralWhich of the following salts is the most basic in aqueous solution 1 Pb CH3COO 2 3 CH3COOK 2 AI CN 3 4 FeCl3 IS HE

Physical Chemistry
Nuclear chemistryNegative Marks 2 in all other cases When photons of energy 4 25 eV strike the surface of a metal A the ejected photoelectrons have maximum kinetic energy T expressed in eV and de Broglie wavelength The maximum kinetic energy of photoelectrons liberated from another metal B by photons of T energy 4 2 eV is T T 1 50 eV If the de Broglie wavelength of these photoelectrons is 22 then which is correct B A A B C D The work function of A is 2 25 eV The work function of B is 3 70 eV TA 2 00eV TB 2 75eV Correct Options A B C D Your Answers AC

Physical Chemistry
EquilibriumBase samples are prepared using NaOH KOH Ba OH 2 Mg OH 2 Al OH separately or as mixture of more than one Calculate the minimum volume of 3 65 w v HCI solution required in L to ensure complete neutralisation in every case possible if total 156 gm of sample is taken

Physical Chemistry
GeneralWhich of the following will decrease with dilution at a given temperature a pH of 10 3 M acetic acid solution b pH of 10 3 M aniline solution c degree of dissociation of 10 3 M acetic acid d degree of dissociation of 10 3 M aniline solution 64 Na MNII CLIf 10 mL of 0 001 M HC

Physical Chemistry
Gaseous and liquid states2 When 3 2 g sulphur is vaporised at 450 C and 723 mm Hg pressure the vapours occupy a volume of 780 mL What is the molecular formula of S vapours a S c S6 d Sg b S4 22

Physical Chemistry
Solutionsetermine What is the boiling point of 1 molal aqueous solution of NaCl K 0 52 K molal 1 99 48 C 2 98 96 C 3 100 52 C 4 101 04 C

Physical Chemistry
GeneralA sample of 4CO was mixed with ordinary 2CO for studying a biological tracer experiment The 10 mL of this mixture at STP possess the rate of 104 disintegration per minute How many milli curie of radioactive carbon is needed to prepare 60 litre of such a mixture

Physical Chemistry
General3 25 min 50 min 4 250 mi For an elementary reaction X g Y g Z g the half life period is 10 min In what period of time the concentration of X will be reduced to 10 of original concentration 1 20 min 2 33 min 3 15 min 4 25 min on is 75 completed in 100 min How long will it take for it s 87 5 completic 0 200 min

Physical Chemistry
GeneralFind the hydrolysis constant of NaX if the dissociation constant of weak acid HX is 2x10 5 Ans 5x10 10

Physical Chemistry
SolutionsWhich of the following solutions will have pH A 2 0 g of NaOH in 500 cm solution C 100 cm solution of 0 1 N Ca OH 13 assuming complete dissociation B 100 cm solution of 0 05 M Ca OH D 4 0 g of NaOH in 500 cm solution

Physical Chemistry
GeneralCations of group IA and group IIA elements are diamagnetic and colourless since their orbitals do not contain odd electrons

Physical Chemistry
GeneralIn a solid AB having the NaCl structure A atoms occupy the every ccp and B atoms are in OHV If all face centred atoms along one of the axes are removed then the resultant stoichiometry of the solid is 1 AB 2 AB 3 A B 4 A B

Physical Chemistry
Atomic StructureThe wavelength of a certain line in the Paschen series in 1094 4 nm of H atom What is the value of high for this line

Physical Chemistry
Atomic Structurecolumn correctly Column I A The radial node of 5s atomic orbital is B The angular node of 3d atomic orbital is C The sum of angular node and radial node of 4d atomic orbital D The angular node of 3p atomic orbital is Column II P 1 Q 4 R 2 S 3

Physical Chemistry
GeneralThe Enthalpy of neutralization of acetic acid and sodium hydroxide is 55 4 kJ What is the enthalpy ionisation of acetic acid 1 5 54 kJ 3 1 9 kJ 2 5 54 kJ 4 1 9 kJ

Physical Chemistry
Gaseous and liquid statesCHOLARSDEN 51 Two glass bulbs A and B are connected by very small tube having a stop cock Bulb A has a volume of 100 ml and contained the gas while bulb B was empty and had a volume of 150 ml on opening the stop cock the pressure of the gas in bulb A will fall down to A 80 B 60 C 40 D 20 the barometric pressure is 750 mmHg The partial pressure of oxygen

Physical Chemistry
Energetics1 litre of water is boiled reversibly inside a pressure cooker The dead weight valve whistle weighing 50 gm lifts up by 2 Cm at 107 C and some steam escapes out The whistle blows 10 times in 5 minutes The Entropy change of the system after 5 minutes is xx 10 3 J K if internal energy change during the process in 10J calculate the value of x Report your answer in single digit obtained by adding up first two digits of your answer 5 Assume steam lost to be of negligible volume and consider each step to be reversible

Physical Chemistry
General9 The number 0 3 g molecule is 1 4 2 NA 3 25 2 NA of electrons in oxygen atom in of Mohr s salt FeSO4 NH4 2SO4 6H O 2 42 NA 4 33 6 NA

Physical Chemistry
Atomic StructureThe number of spherical nodes in 3p orbitals is b three a one c two d zero

Physical Chemistry
Generaliii 3 4 5 6 7 181 9 10 The total charge coulombs required for complete electrolysis is A 24125 B 48250 C 96500 D 193000 A student performs a titration with different burettes and finds titre values of 25 2 mL 25 25 mL and 25 0 mL The number of significant figures in the average titre value is JEE 2010 3 163 Reaction of Br with Na CO3 in aqueous solution gives sodium bromide and sodium bromate with evolution of CO gas The number of sodium bromide molecules involved in the balanced chemical JEE 2011 4 180 equation is JEE 2007 4 162 Dissolving 120 g of urea mol wt 60 in 1000 g of water gave a solution of density 1 15 g mL The JEE 2011 3 160 molarity of the solution is A 1 78 M B 2 00 M C 2 05 M D 2 22 M 29 2 w w HCI stock solution has a density of 1 25 g mL The molecular weight of HCI is 36 5 g mol The volume mL of stock solution required to prepare a 200 mL solution of 0 4 M HCl is JEE 2012 4 136 For the reaction I CIO3 H SO4C HSO4 1 The correct statement s in the balanced equation is are A Stoichiometric coefficient of HSO is 6 B lodide is oxidized C Sulphur is reduced D H O is one of the products JEE Advanced 2014 3 120 A compound H X with molar weight of 80 g is dissolved in a solvent having density of 0 4 g ml Assuming no change in volume upon dissolution the molality of a 3 2 molar solution is JEE Advanced 2014 3 120 and solvent The mole fraction of a solute in a solution is 0 1 At 298 K molarity of this solution is the same as its molality Density of this solution at 298 K is 2 0 g cm The ratio of the molecular weights of the solute MWsolute MW solvent JEE Advanced 2016 3 124 is The order of the oxidation state of the phosphorus atom in H PO2 H3PO4 H3PO3 and H4P O6 is JEE Advanced 2017 3 122 B H3PO4 H4P206 H3PO3 H PO2 D H3PO3 H PO2 H3PO4 H P O6 A H3PO4 H3PO2 H3PO3 H4P2O6 C H PO H3PO3 H4P2O6 H3PO4 PART II JEE MAIN AIEEE PROBLEMS PREVIOUS YEARS m are present in

Physical Chemistry
Surface chemistry0 Which of the following has minimum protecting power 1 Gelatin Gold no 0 01 2 Dextrin Gold no 15 3 Potato starch Gold no 25 4 Albumin Gold no 0 25

Physical Chemistry
Electrochemistryand equilibrium constant of the reactions 5 Write the Nernst equation and emf of the following cells at 298 K i Mg s Mg2 0 00TM Cu 0 0001 M Cu s ii Fe s Fe 0 001M H 1M H g 1bar Pt s iii Sn s Sn 0 050 M H 0 020 M H g 1 bar Pt s iv Pt s Br l Br 0 010 M H 0 030 M H g 1 bar Pt s

Physical Chemistry
GeneralWhich of the following is are correct A At 75 C ionic product of water is more than 1 0 10 4 M B At 25 C degree of dissociation of water is 1 8 x 10 7 C At 75 C water is acidic D PH pOH 14 at all temperature

Physical Chemistry
Equilibrium85 100 mL of 0 02 M benzoic acid pK 4 20 is titrated using 0 02 M NaOH pH after 50 mL and 100 mL of NaOH have been added are a 3 50 7 b 4 2 7 c 4 2 8 1 d 4 2 8 25

Physical Chemistry
Electrochemistry4 ACE Aluminium oxide may be electrolysed at 1000 C to furnish aluminium metal Atomic mass 27 amu 1 Faraday 96 500 Coulombs The cathode reaction is Al 3e AP To prepare 5 12 kg of aluminium metal by this method would require 1 5 49 10 C of electricity 3 5 49 10 C of electricity Q mFZ M 2 1 83 10 C of electricity 4 5 49 10 C of electricity MAINS 2005

Physical Chemistry
Chemical kineticsPure water freezes at 273 K and 1 bar The addition of 34 5 g of ethanol to 500 g of water changes the freezing point of the solution Use the freezing point depression constant of water as 2 K kg mol The figures shown below represent plots of vapour pressure V P versus temperature T molecular weight of ethanol is 46 g mol 1 Among the following the option representing change the freezing point is A B V P bar V P bar Ice 270 273 Ice 1 Water Ethanol 1 I T K I I 270 273 Water T K Water Water Ethanol V P bar V P bar Ice Ice I I 1 271 273 T K 1 271 Water I Water Ethanol Water 1 IWater Ethanol 273 T K 2017

Physical Chemistry
Gaseous and liquid states0 10 1 0x10 2 kg of hydrogen and 6 4x10 2 kg of oxygen are contained in a 10x10 3 m flask at 473 K Calculate the total pressure of the mixture If a spark ignities the mixture What will be the final pressure

Physical Chemistry
Solid stateThe edge length of face centred cubic unit cell having rock salt structure is 508 pm If the radius of the c is 110 pm the radius of the anion is 4 398 pm 1 144 pm 2 288 pm 3 618 pm

Physical Chemistry
EquilibriumThe reaction cis Cr en OH 2 trans Cr en OH 2 K is first order in both directions At 25 C the equilibrium constant is 0 1 and the rate consta k is 2 10 4s In an experiment starting with the pure cis form how long would it take for half t equilibrium amount of the trans isomer to be formed In2 0 693 k

Physical Chemistry
Solid stateAssertion A particle present at the corner of the face centred unit cell has 1 8th of its contribution of the unit cell Reason In any space lattice the corner of the unit cell is always shared by the eight unit 2 B 3 C 4 D cells 1 A

Physical Chemistry
GeneralThiosulphate reacts differently with iodine and bromine in the reactions given below 2S O3 1 S 0 21 2 S O3 2Br 5H O2SO2 2Br 10H Which of the following statements justifies the above dual behaviour of thiosulphate 201 a b Bromine is a stronger oxidant than iodine Bromine is a weaker oxidant than iodine c Thiosulphate undergoes oxidation by bromine and reduction by iodine in these reactions Bromine undergoes oxidation and iodine undergoes reduction in these reactions d

Physical Chemistry
SolutionsFor a dilute solution containing 2 5 g of a non volatile non electrolyte solute in 100 g of water the elevation in boiling point at 1 atm pressure is 2 C Assuming concentration of solute is much lower than the concentration of solvent the vapour pressure mm of Hg of the solution is take K 0 76 K kg mol A 724 C 736 B 740 D 718 2012 Fe CN Mol Wt 329 in 100 g of

Physical Chemistry
Atomic StructureWhich general electronic configuration of the element does not represent a non metal metel is in s p 1 ns np 2 ns n 1 d 10 np5 3 ns 2 n 1 d1 10 4 ns n 1 0 10 np1 6 d black metal

Physical Chemistry
Gaseous and liquid statesExample 19 7 One end of a copper rod of length 1 m and area of cross section 4 0 104 m is maintained at 100 C At the other end of the rod ice is kept at 0 C Neglecting the loss of heat from the surroundings find the mass of ice melted in 1 h Given kcu 401 W m K and L 3 35 x 105 J kg

Physical Chemistry
GeneralTwo elements X Atomic weight 75 and Y Atomic weight 16 combine to give a compound having 75 8 of X The empirical formula of compound is 1 XY 2 X Y 3 X Y 4 X Y

Physical Chemistry
GeneralMultiple Choice Questions 1 The squirrel wore a a tail c a coat FIN b a wig d a sm

Physical Chemistry
EquilibriumFor the reaction 2CO O 2CO AH 560 kJ Two moles of CO and one mole of O are taken in a container of volume 1 L They completely form two moles of CO2 the gases deviate appreciably from ideal behaviour If the pressure in the vessel changes from 70 to 40 atm find 2006 the magnitude absolute value of AU at 500 K 1 L atm 0 1 kJ

Physical Chemistry
EquilibriumThe electrochemical cell shown below is a concentration cell MM saturated solution of a sparingly soluble salt MX M 0 001 mol dm3 M he emf of the cell depends on the difference in concentrations of M ions at the two electrodes The emf of the cell at 298 K is 0 059 V 2012 The value of AG kJ mol for the given cell is take 1F 96500 C mol B 5 7 D 11 4 A 5 7 C 11 4 The solubility product Ksp mol dm of MX at 298 K based on the information available for the given concentration cell is take 2 303 x R x 298 F 0 059 V B 4 x 10 15 D 4 x 10 A 1 x 10 15 C 1 x 10 12

Physical Chemistry
EnergeticsA radiation of 253 7 nm strikes the surface of a metal and ejected electrons are stopped by 0 24 eV The work function of the metal is A 2 65 eV C 4 65 eV B 3 65 eV D 5 65 eV

Physical Chemistry
Chemical kineticsA definite volume of H O under going spontaneous decomposition required 25 ml of standard permanganate solution for titration After 10 and 20 minutes respectively the volumes of permanganate required were 15 ml and 9 ml respectively Calculate the fraction of H O decomposed after 25 minutes Given 0 6 2 5 0 28 K Xa p 12 21 P q 2

Physical Chemistry
Chemical kineticsFor a reaction AB C it was found that at the end of 10 min from the start the total optical rotation of the system was 50 and when the reaction is complete it was 100 Assuming that only B and C are optically active and dextro rotatory the rate constant of this first order reaction would be a 0 069 min c 6 9 min a b 0 69 min 1 d 6 9 x 10 2 min

Physical Chemistry
GeneralA first order reaction is carried out with an initial concentration of 10 mol per litre and 80 of the reactant changes into the product Now if the s reaction is carried out with an initial concentration of 5 mol per litre for the same period the percentage of the reactant changing to the product is 2 80 1 40 3 160 4 Cannot be calculated