Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
GeneralA and 1 55 g of B molar weight is 160 g mol are placed in a 1 00 L reaction vessel and sealed the mixture reacts and the following equilibrium is established 2A 9 B g 2C 9 At 25 C the equilibrium pressure of C is 0 264 atm What is K

Physical Chemistry
Energeticstion is false but reason is true Assertion Increasing pressure of pure water decreases its freezing point Density of water is maximum at 273 K Reason AIIMS 2003 a If both assertion and reason are true and the reason is the correct explanation of the assertion b If both assertion and reason are true but reason is not the correct explanation of the assertion c If assertion is true but reason is false d If the assertion and reason both are false e If assertion is false but reason is true

Physical Chemistry
GeneralIn two vessels of 1 L each at the same temperature 1 g of DCE 2007 H and 1 g of CH4 are taken for these a Vs values will be same rms b Kinetic energy per mol will be same c Total kinetic energy will same d Pressure will be same

Physical Chemistry
Equilibrium70 Determine the value of equilibrium constant K for the reaction A g B g 2AB g if 10 moles of A2 15 moles of B2 and 5 moles of AB are placed in a 2 litre vessel and allowed come to equilibrium The final concentration of AB is 7 5 M a 4 5 b 1 5 c 0 6 11 1 1 d None of these

Physical Chemistry
Atomic StructureIf W is atomic weight and N is the atomic number of an element then CPMT 1971 80 89 a Number of e W N b Number of on W N c Number of H W N d Number of on N

Physical Chemistry
SolutionsThe freezing point of a solution of acetic acid in benzene is 277 4 K Acetic acid partially dimeris Melting point of benzene is 278 4 K AusionH 10 042 kJ mole mass fraction of acetic acid in solution is 0 015 The initial moles of acetic acid in solution before dimerisation is x The degree of dissociation of dimerisation of CH3COOH in solution is y Then x y is

Physical Chemistry
GeneralFor a reaction of order n what is the relationship between t3 4 and where t is the time required for concentration C to become 1 4 C and Care the values of the reactant concentration at the start and after time t respectively 1 13 4 1 2 2 1 1 3 3 4 1 2 2 1 11 2 3 4 2120 1 1 4 3 4 1 2 2 1 1

Physical Chemistry
Atomic StructureA 100 watt bulb emits monochromatic light of wavelength 400 nm Calculate the number of photons emitted per second by the bulb 20 1 a 3 10 0 s c 2 1020 S 1 b 2 x 10 20 1 d 1 x 10 20 S 1

Physical Chemistry
Equilibrium73 180 Mark for Review When initial concentration of a reactant is doubled in a reaction its half life period is not affected The order of the reaction is Zero 02 4 hr m More than zero but less than first First

Physical Chemistry
Chemical kineticsIf 40 of first order reaction was completed in 50 minutes then 50 of the same reaction would be completed in approximately Given log 3 0 47 log 5 0 7 79 minute 65 minute 61 minute

Physical Chemistry
Gaseous and liquid statesFor every 33 feet beneath the surface of the ocean the pressure increases by 1 0 atm A fish having had too rich a lunch burps and the resultant bubble rises toward the surface When released at a depth of 198 ft the bubble had a volume of 12 mL and a temperature of 3 0 C The conditions at the surface of the ocean are 23 C and 0 97 atm of pressure What will the volume of the bubble be when it reaches the surface

Physical Chemistry
General3 C Energy of the electron in Hydrogen atom is given by a En c En 131 38 2 n 1313 3 2 n kJ mol b E AMU Engg 2002 131 33 kJ mol kJ mol d E n 313 13 2 n kJ mol P31

Physical Chemistry
General10 mole of ideal gas expand isothermally and reversibly from a pressure of 10 atm to 1 atm at 300 K What is the largest mass which can lifted through a height of 100 meter a 31842 kg b 58 55 kg c 342 58 kg d None of these 0

Physical Chemistry
General2 A device for sustained drug delivery is of the shape of a thin slab Both the top and bottom surface with an area of 1 5mm each are covered by polymeric membranes to control diffusion The core is a drug reservoir with very high drug concentration at 20 mg ml The device has a constant drug release rate of 2 g day a What is the permeability of the membrane for the drug State all assumptions in your calculation

Physical Chemistry
GeneralTwo drugs have a selectivity factor a 1 08 and capacity factors k 5 0 and k 5 4 If the plate height is 0 20 mm how long must the column be for a resolution of 1 5 A 22 3 m B 87 4 m 34 5 m D 17 3 m

Physical Chemistry
Chemical kineticsIf rate constant of reaction becomes twice when temperature increases from 300 K to 310 K then activation energy in Joules of the reaction is Given log 2 0 30 O2 303 90 R O2 303 31 R O 2 303 x 90 31 2 303 x 90 x 31 R

Physical Chemistry
Generala 8 Write a detailed stepwise mechanism to explain ONLY ONE of the following reactions 5 points DO NOT DO MORE THAN ONE acetic acid OH HO isoamyl alcohol H O heat bu isoamyl acetate 9

Physical Chemistry
Solutionsphase will be always rich in the component which is more vo The vopour pressure of pure benzene at a certain temperature is 0 850 bar A non volatile non electrolyte solid weighing 0 5g when added to 39 0 g of benzene molar mass 78g mol vapour pressure of the solution then is 0 845 bar what is the molar mass of the solid substance
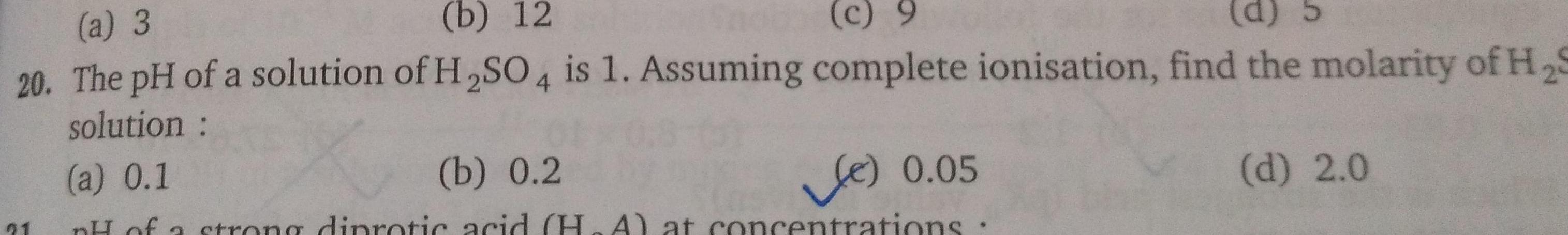
Physical Chemistry
Generala 3 b 12 c 9 d 20 The pH of a solution of H SO4 is 1 Assuming complete ionisation find the molarity of H S solution a 0 1 b 0 2 Le 0 05 pH of a strong diprotic acid H A at concentrations 21 d 2 0

Physical Chemistry
GeneralAt constant volume for a fixed number of a moles of a gas the pressure of the gas increases with the rise in temperature due to 1 Increase in average molecular speed 2 Increase in rate of collisions amongst 3 Increase in molecular attraction 4 Increase in mean free path

Physical Chemistry
EnergeticsThe difference in free energy of the reaction when all the reactants and products are in STP 1 atm and 298K is AG and ke and kp are the thermodynamics equilibrium constants of the reaction The relation between them is as follows 4 5 AG 2 303 RT log Kc and AG 2 303RT log Kp in case of ideal gas Which of the following statement is correct for a reversible process in state of equilibrium A AG 2 303 RT log K B AG 2 303 RT log K C AG 2 303 RT log K D AG 2 303 RT log K At 490 C the value of equilibrium constant k is 45 9 the reaction H g 1 g 2HI g Calculate the value of AG for the reaction at that temperature A 3 5 kcal B 3 5 kcal C 5 79 kcal D 5 79 kcal

Physical Chemistry
Solutions20 gm of binary electrolyte mol wt 100 was dissolved in 500 gm water Freezing point of solution was found to be 0 74 C K 1 86 K molality What is the degree of dissociation of the electrolyte

Physical Chemistry
GeneralA chemist wants to prepare phosgene COCI by the following reaction CO g Cl g COCI g He places 2 20 g of chlorine Cl and an equal molar amount of carbon monoxide CO into a 10 00 L reaction vessel at 395 C After the reaction comes to equilibrium he adds another 2 20 g of chlorine to the vessel in order to push the reaction to the right to g more product What is the partial pressure of phosgene when the reaction again comes to equilibrium K 1 23 x 10 Partial pressure atm

Physical Chemistry
General6 In Partition coefficient experiment aqueous layer comes below n Butanol layer because O O O O a The density of water is less than n Butanol b Acetic acid is more soluble in n Butanol layer c The density of water is more than n Butanol d Acetic acid is more soluble in aqueous layer

Physical Chemistry
GeneralAs the volume of HCl and NaOH is increased containing 25 mL 0 5M acetic Acid Acetate Buffer describe the effect on the pH Did the buffer stop working at some point If so at what volume of acid and base did the buffer fail Why

Physical Chemistry
EnergeticsThe combustion of fuel in a piston cylinder causes an internal energy change of 2 843E3 kJ The components surrounding the cylinder absorb 9 566E2 kJ of heat How much work can be done by the fuel on the piston in the cylinder

Physical Chemistry
EquilibriumFor each of the salts on the left match the salts on the right that can be compared directly using Ksp values to estimate solubilities If more than one salt on the right can be directly compared include all the relevant salts by writing your answer as a string of characters without punctuation e g ABC 1 calcium chromate 2 manganese II hydroxide Ksp Write the expression for K in terms of the solubility s for each salt when dissolved in water calcium chromate manganese II hydroxide A Ag SO4 B CaSO4 C ZnS D CrPO4 Ksp Note Multiply out any number and put it first in the Ksp expression Combine all exponents for

Physical Chemistry
Electrochemistry25 g of a metal is deposited on cathode during electrolysis of metal nitrate solution by a current of 5 ampere passing for 4 hours If atomic weight of metal is 200 the valency of metal in metal nitrate is n What is n

Physical Chemistry
Equilibrium87 In which of the following equilibrium change in volume of the system does not alter th number of moles a N g O g 2NO g c N g 3H g 2NH3 g b PC15 g PC13 g Cl g d SO Cl g SO g Cl g

Physical Chemistry
Equilibrium82 following eq N 04 8 2NO g Then the select the correct graph which shows the variation in concentrations of N 04 against concentrations of N 04 VC V L b c d N 04 N 04 N 04 N 04

Physical Chemistry
GeneralMark O if the statement is true X if false For the false statements correct them a Carbon coked catalysts can be reused after treatment with hot steam b For a certain catalytic reaction where A is converted to either B and C if the differential selectivity with respect to product B is 0 5 the production rate of C is twice faster than that of A c Attrition is a type of irreversible deactivation of catalysts d The most appropriate type of reactor in the conversion of methane to hydrogen by reacting with gaseous water is single pass fixed bed reactor

Physical Chemistry
GeneralA 0 846 M KMnO4 solution was used to determine the hydrogen peroxide H O2 MM 34 016 content in a 25 48 mL of peroxide solution It took 13 28 mL of the KMnO4 solution to reach the endpoint What is the percentage w v of H O2 in the sample The reaction is 2 5 H O 2 MnO4 6 H 5 O 2 Mn 8 H O

Physical Chemistry
GeneralRefer to the given graph plotted for three different fabric pieces of equal size P Q and R on the basis of their water absorbing capacity and select the correct statement Amount of water absorbed 0 Fabric A P is made up of fibres obtained from Gossypium plant whereas R is made up of fibres obtained from an insect BQ is made up of protein fibres obtained from an insect whereas R is made up of cellulose fibres obtained from flower of a plant C R is made up of fibres obtained from plant stem by the process of retting whereas P is made up of fibres obtained from plant seed by the process of ginning

Physical Chemistry
GeneralA solution containing 0 100 mol of hydrochloric acid is added to 8 43 g of magnesium carbonate MgCO3 s 2HCl aq MgCl aq CO g H O l a What is the total volume at r t p of carbon dioxide formed M MgCO3 84 3 A 2 40 dm B 1 20 dm C 2400 dm D 1200 dm Molar volume of a gas at r t p 24 0 dm mol

Physical Chemistry
GeneralHow many of the following compounds are more acidic than water OH a OH 0 NO c i methanol NO g ethanol h isopropyl alcohol OH d 0 NO OH 0 min sec e OH f OCH OO CH

Physical Chemistry
Chemical kineticsAt certain temperature the half life period in the thermal decomposition of a gaseous substance as follows P mmHg 500 t 2 in min 235 Find the order of reaction Given log 23 5 1 37 log 95 1 97 1 1 250 950 2 2 3 2 5 4 3

Physical Chemistry
Equilibrium3 Calculate non ionized the ratio of ionized and forms of the drug novocaine in the stomach pH 1 98 and the intestine pH 8 98 if its pK 8 98 Where will the drug be absorbed in the stomach and or intestines 10 01

Physical Chemistry
GeneralBased on the equation CH4 g 202 g CO2 g 2H O g what is the change in internal energy if 4 5 grams od CH4 was oxidized at 25 C Select one O a 190 15 kJ b 195 68 kJ C 200 63 kJ d 199 24 kJ AH 802 kJ

Physical Chemistry
Electrochemistry800 milliampere of current is passed aqueous for a at anode sol period of of cusof using two 20 minutes predict products cathode estimate quantity of products 63 6 At uit of cu is through an Pt electrode

Physical Chemistry
GeneralA 0 3146 g sample of a mixture of NaCl s and KBr s was dissolved in water The resulting solution required 46 00 ml of 0 08765 M AgNO aq to precipitate the CI aq and Br aq as AgCl s and AgBr s Calculate the mass percentage of NaCl s in the mixture

Physical Chemistry
EquilibriumWith calcium hydroxide the reactions Ca OH 2 s Ca2 2OH and Ca2 OH Ca OH have ksp 5 3 x 10 6 and pK1 1 55 respectively A How many moles of calcium hydroxide are dissolved in 125mL of water ksp 5 3 x 10 6

Physical Chemistry
Atomic StructureThe density of a solution was determined from the mass of a 10 mL sample of the solution on an analytical balance For this experiment each 10 mL sample was dispensed from one of three different 10 00 mL volumetric pipettes A total of twelve measurements were made where four samples were dispensed from each pipet The calculated density values from each pipet are reported below Glassware Pipet 1 Pipet 2 Pipet 3 1 54 g mL 1 46 g mL 1 43 g ml be tabulated values Calculated Density of Solution 1 55 g mL 1 54 g mL 1 55 g mL 1 41 g mL 1 49 g mL 1 44 g ml 1 50 g mL 1 42 g mL 1 53 g mL which of the ninets give s the most precise results

Physical Chemistry
EquilibriumThe equilibrium of a pure water dissociating into its ions has a relation time of around 57 microseconds at 25 C Find the rate constants of both the forward and reverse reaction The density of water is 1 00 g mL

Physical Chemistry
GeneralConsider the following reaction 3 A 7 B 4D 2E If 17 moles of A 42 moles of B and 33 moles of D are present Calculate moles of E produced at 50 completion of reaction O 5 67 O 8 5 O 11 33 O 9 7

Physical Chemistry
Gaseous and liquid statesSide arm of an open end mercury manometer is attached to a gaseous system The level of mercury in the arm attached to the system is 125 mm lower than the open end arm What is the pressure of the gaseous system if atmospheric pressure is 760 mm Hg

Physical Chemistry
Equilibrium21 pH of a strong diprotic acid H A at concentrations i 10 4 M ii 104 N 22 are respectively 3 7 and 4 0 Calcium 1 b 4 and 3 7 c 4 and 4 XOH d 3 7 and 3 KA

Physical Chemistry
Chemical kineticsThe suggested mechanism of a reaction is a A B D fast b A D 2C slow Write the balanced equation of the reaction if it experimentally deduced rate equation is rate k A B Find the intermediate formed during the course of the reaction Does the predicted rate law from the mechanism match the experimental rate law

Physical Chemistry
General13 9 g of FeSO4 xH O crystals were dissolved in a dilute sulphuric acid and volume made up to 1000 ml 25 ml portion of this solution required 50 ml and 0 005 M KMnO4 solution to reach the end point The value of x to the nearest integer is Fe 56 S 32 0 16 g mol Answer Enter your answer here Back Space

Physical Chemistry
GeneralFT IR cm 1 740 H NMR 84 35 1H quartet 4 25 2H quartet 1 8 3H doublet 1 3 3H triplet 1 C NMR DEPT CH CH3 CH 8 62 1 40 1 22 1 15 Mass spectrum m e 180 182 M 152 154 107 109 determine the structure of C5H902Br

Physical Chemistry
General36 The initial concentration of the reactant in a first order reaction is a It takes time t for the completion of n th fraction of the reaction Will it take time 2t for the completion of the same fraction if the initial concentration of the reactant is made twice