Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Equilibrium71 At 87 C the following equilibrium is established H g S s RO H S g K 7 x 10 If 0 50 mole of hydrogen and 1 0 mole of sulphur are heated to 87 C in 1 0 L vessel what will be the partial pressure of H S at equilibrium a 0 966 atm b 1 38 atm c 0 0327 atm d 1 atm d into an quacuated chamber and comes to equilibrium at 247 C and 2 0 n DOL

Physical Chemistry
Atomic Structure18 The radius of the first Bohr orbit of a hydrogen atom is 0 53 x 108 cm The velocity o the electron in the first Bohr orbit is A 2 188 x 108 cm s 1 C 1 094 x 108 cm s RIGH NIKE A 2 188 x 108 cm s C 1 094 x 108 cm s 1 B 4 376 108 cm s D 2 188 x 10 cm s A 0 53 x 108 cm 1 B D GUGE OK K 4 376 x 108 cm s 1 2 188 x 10 cm s

Physical Chemistry
GeneralCOOH OH CH CO OH Salicylic acid Common name of the compound formed in by above reaction will be O Aspirin Oil of winter green Salol

Physical Chemistry
General1 i ii Features a Thick waxy covering b Large fleshy stems c Spikes d Shallow roots that spread out a long way Define endangered 3 How the feature helps the cactus survive in the desert

Physical Chemistry
GeneralIf a hydrate of Ba CIO4 2 is heated to 250 C all the water of hydration is lost On heating a 1 687 g sample of the hydrate 1 453 g of Ba CIO4 2 remains How many molecules of water occur per formula unit of Ba CIO4 2

Physical Chemistry
Chemical kinetics122 For a reaction NO g O2 g 2NO g Rate k NO 0 if the volume of the reaction vessel is double the rate of reacti a will diminish to 1 4 of initial value b will diminish to 1 8 of initial val c will grow 4 times d will grow 8 times

Physical Chemistry
EquilibriumA 2 lit flask initially containing one mol of each CO and H O was sealed and heated to 700 k where the following equilibrium was established CO g H O g CO g H g Kc 9 2 Now the flask was connected to another flask containing some pure CO g at same temperature and pressure by means of a narrow tube of negligible volume When the equilibrium was restored moles of CO was found to be double of its moles at first equilbrium Determine volume of CO g flask

Physical Chemistry
Chemical kineticsfor a reversible first order reaction A K Kb B k 10 2sec and Bea A eq 4 A 0 01 M and B 0 The concentration of B after 30 sec is 2 48 10 M The value of x is Given e 0 375 0 69

Physical Chemistry
Chemical kineticsWhich of the following is are correct statement s according to the Arrhenius equation A For given reaction the pre exponential factor has same units as that of rate constant B Higher the magnitude of activation energy greater is the temperature dependence of the rate constant C Rate constant increases with increase in temperature This is due to a greater number of collisions of molecule whose energy exceeds the activation energy Pato constant k F RT

Physical Chemistry
GeneralThe resistance of a 0 1 M solution of a weak acid HA in a cell was found to be 600 ohm What will be its pH at the same concentration Given cell constant 0 936 cm 1 M NaCl 126 S cm mol A M HCI 426 S cm mol A M NaA 90 S cm mol A 3 log 4 C 5 log 4 B 2 log 4 D 6 log 4

Physical Chemistry
General3 Write chemical equations for the following reactions a Calcium hydroxide reacts with nitric acid b Acetic acid reacts with calcium hydroxide e Hydrochloric acid reacts with sodium hydroxide d Ammonium hydroxide reacts with sulphuric acid ca x z

Physical Chemistry
Chemical Bonding3 C O F CI NO CN 1 The overall formation constant of the Co NH 2 ion in aqueous solution is 105 and the standa potentials for the reduction of Co aq and Co NH 1 aq are as follows Co aq eCo aq E 1 9V Co NH3 aq e Co NH3 aq E 0 1V Calculate the nearest overall formation constant of the Co NH3 ion www 1 1030 2 1033 3 1035 4 1038 iment corresponding

Physical Chemistry
GeneralThe van t Hoff factors for mercurous chloride and mercuric chloride are respectively unity and greater than unity Which of the following statements is true about two chlorides 0000 Mercuric chloride is covalent Dissociation of HgCl2is incomplete while mercurous chloride is complete Mercurous chloride is sparingly soluble in water Both mercuric and mercurous chlorides are covalent

Physical Chemistry
Equilibrium6 a For the equilibrium COCl2 g CO g Cl2 g Kp is 8x10 9 at 127 C Calculate the degree of dissociation of phosgene and AH for the reaction at that temperature Given that total pressure is 2 atm and AS 400K 30 cal deg mol 4 CU 2012 1 b WBSU 2015 6 a

Physical Chemistry
Generalsec Which of the following alkene will not be formed on dehydration of 3 methylhexan 2 ol

Physical Chemistry
EquilibriumHistidine is a common amino acid found in many proteins and other natural sources There are 3 deprotonation steps of this substance with three acid dissociation constants K 2x10 2 K 9 5x10 7 and K3 8 3x10 10 when ionic strength is 0 1 as follows a If the pH of a histidine solution is 6 05 which form of histidine is present in the solution b How many ml of 0 1135M NaOH are needed to get a buffer solution with 70 nl

Physical Chemistry
Equilibrium68 COCl gas dissociates according to the equation COCl g CO g Cl g When heated to 700 K the density of the gas mixture at 1 16 atm and at equilibrium is 1 16 g litre The degree of dissociation of COC12 at 700 K is a 0 28 b 0 50 c 0 72 d 0 42

Physical Chemistry
ElectrochemistryThe conductivity of 0 0099 mol L benzoic acid is 4 95 x 10 5 S cm Its K x 10 6 dissociation constant If L for benzoic acid is 500 S cm mol at th temperature then find x Answer in nearest integer

Physical Chemistry
GeneralWBSU Year 2018 5 a How will the advancement of reaction S change for N2O4 g 2NO2 g when i 5 mol of Ar introduced keeping mixture at constant P ii 5 mol of Ar introduced keeping mixture at constant V 4

Physical Chemistry
EquilibriumWhich of the following is are correct for 0 1 M BOH solution K 10 5 pH of solution is 11 OH concentration is 10 3 mol L it s salt with HCl i e BCI forms acidic solution in water Phenolphthalein indicator can be used LIOL

Physical Chemistry
GeneralRead the information about all the makers of the Indian Constitution given in the side columns here You don t need to memorise this information Just give examples from these to support the following statements 1 The Assembly had many members who were not with the Congress 2 The Assembly represented members from different social groups 3 Members of the Assembly believed in different ideologies
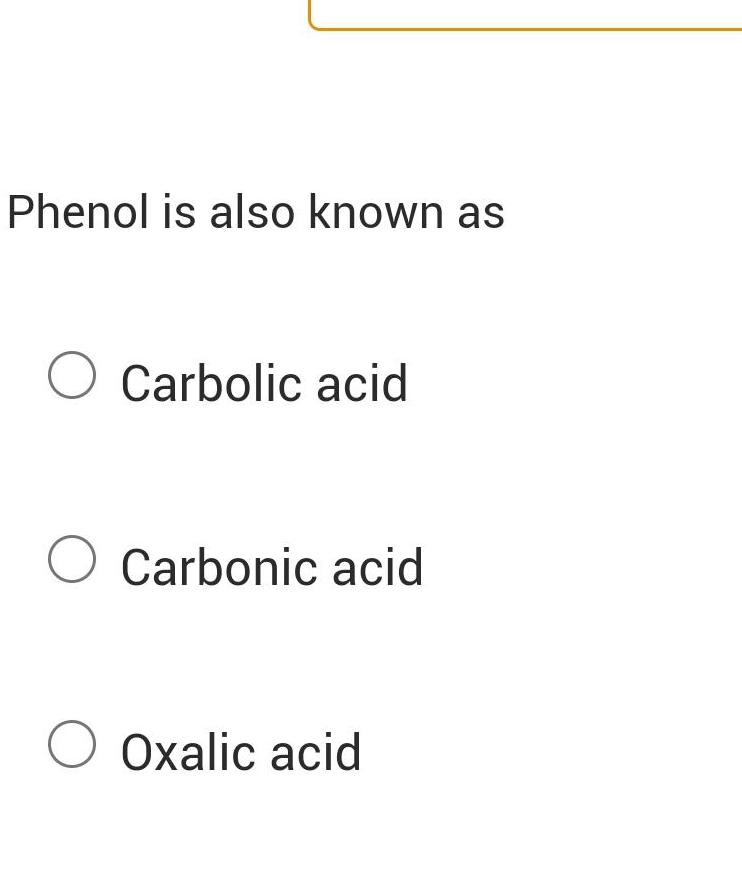

Physical Chemistry
GeneralPhenol pKa 9 95 and sodium bicarbonate pKa 10 2 react to form an equilibrium with conjugate base of phenol and carbonic acid pKa 6 4 What is the Keq OA 1 58 x 10 4 OB 0 000282 OC 3 80 OD 2 51 x 10 4 OE 1 58 x 10 4 OF 1 58

Physical Chemistry
GeneralThe correct statement about SO3 and ClOzis i Both are isoelectronic iii Both are not isoelectronic O O ii ii O i ii i iv iii iv ii Both are isostructural iv Both are not isostructural

Physical Chemistry
Gaseous and liquid statesb For the complete combustion of methane gas according to the equation CH4 g O2 g H20 g CO2 g The density of CO2 gas fully collected in a cylinder with a piston fixed at 273k and 760 mm HG is 1 788 g L What is the molecular weight of the gas

Physical Chemistry
GeneralYou are a biotechnologist designing a new elimination reaction Based on the retrosynthetic analysis you completed there s a possibility that you will have E and E competing mechanisms in your reaction What is the best way to set up the laboratory experiment so that you can differentiate between E and E mechanisms Specify how would you experimentally determine the mechanism of your new reaction 5 marks

Physical Chemistry
EquilibriumAir at 20 C in a cylinder 0 15 m high and sealed by a piston is compressed reversibly and adiabatically to 2 cm Calculate the final air temperature answers below in degrees Celsius For air Cp 1 005 J g K and cv 0 718 J g K a 283 O b 299 C 324 d 369 e 383 f 401 9 444 h 461

Physical Chemistry
EquilibriumSuppose a 500 mL flask is filled with 0 80 mol of H O 1 8 mol of CO and 0 20 mol of H The following reaction becomes possible CO g H O g CO g H g The equilibrium constant K for this reaction is 0 179 at the temperature of the flask Calculate the equilibrium molarity of CO Round your answer to two decimal places

Physical Chemistry
GeneralIf a 10 gm sample of dry ice is kept in a bottle of 2 L capacity at 27 C and at a time pressure is found to be 760 mm of Hg then find the approximate weight of dry ice left Only One Correct Answer A B 6 4 gm 3 2 gm 2

Physical Chemistry
Gaseous and liquid states4 0 g of argon has pressure P and temperature T K in a vessel On keeping the vessel at 50 higher temperature 0 8 g of argon was given out to maintain the pressure P The original temperature was A 73 K B 100 K C 200 K D 510 K

Physical Chemistry
EquilibriumWhat will be the total pressure of the gaseous mixture at equilibrium if AB is 20 dissociated according to the following reaction AB g equilibrium 1 4 Kp 2 7Kp1 2 3 2K1 2 1 2 A g 2B g Kp represents the constant of the reaction

Physical Chemistry
EquilibriumWBS Year 3 e When N2 and H in the ratio 1 3 are allowed to react at 100 atm and 200 C it was found that the conversion to NH3 was 25 of original volume calculate Kp for the reaction N2 g 3H2 g 2NH3 g 4

Physical Chemistry
GeneralIve done practice problems where these questions were worded differently and between 2 answers for them If someone can explain what the answer is so I can understand it for my next exam 14 Which of the following is the B D gulopyranose HO A B C D E HO A OH 2 1 0 OH OH 1 2 HO H B OH OH OH HO HO C OH OH OH HO H HO D OH OH HO H E OH 15 What is the overall net charge on the pentapeptide CSKKG at pH 8 2 OH OH

Physical Chemistry
Atomic StructureDIRECTION Fill up the table below Check to determine the type of force that most evident for each substance SUBSTANCE H F HHH H C C C H 1 II HHH 0 H H N H H KBr in H O POLARI NONPOLAR DIPOLE HYDROGEN ION BOND LONDON DISPERSION DIPOLE FORCES FORCES DIPOLE FORCES

Physical Chemistry
Solutionsglucose solu 2 If mole fraction of the solvent in solution decreas then 1 Vapour pressure of solution increases 2 B P decreases 3 Osmotic pressure increases 4 All are correct IS00

Physical Chemistry
Electrochemistry9 One litre of a gas weighs 2 g at 300 K and 1 atm pressure If the pressure is made 0 75 atm at which of the following temperatures will one litre of the same gas weigh one gram a 450 K b 600 K c 800 K d 900 K

Physical Chemistry
ElectrochemistryWhich of the following is wrong 1 Ecell R P of Cathode R P of Anode 2 Ecell O P of Anode O P of Cathode 3 Ecell O P of Anode O P of Cathode 4 Ecell O P of Anode R P of Cathode

Physical Chemistry
GeneralCan we say in adiabatic process if change i n heat is zero then change in temp will ulti mately be zero as zeroth law says that energy flows in for m of heat when there is a temp differnce h ence if there is no change in heat there will be no temp difference

Physical Chemistry
Chemical BondingWe know the reaction between Zinc and sul phuric acid and as a product it releases the H2 gas Now if we take some amount of so dium hydroxide in place of sulphuric acid an d react it with zinc what will be the change i n amount of hydrogen gas produced a Same amount of H2 gas is evolved b H2 gas is not evolved c The amount of H2 gas evolved is much I ess d In place of H2 gas 02 gas is evolved

Physical Chemistry
SolutionsA solid substance A is soluble in water to the extent of 10 mg mL of water at 250C and 100mg mL of water at 1000C You have a sample that contains 100 mg of A and 25 mg of an impurity B A and B have the same solubility behavior If one 10mL portion of water was used for the recrystallization what was the recovery of A A cannot be determined B 90 C 70 D 0

Physical Chemistry
Generalgiven setup burner test tube stand the chemicals used and their pro test Tube jas jar 75 through of water LABORATORY PREPERATION OF OXYGEN A Potassium chlorate and Manganese dioxide 5 1 B Potassium chlorate and Nickel S 1 C Potassium chlorate and Manganese dioxide Is inickel 1 5

Physical Chemistry
GeneralState whether each of the following statements is TRUE or FALSE and explain your answer a A MALDI TOF mass spectrometer with the upper mass limit of m z 1500 is not able to analyze a protein of mass 15 000 Da 4 marks b ESI is preferred over MALDI in some chemical analyses 4 marks

Physical Chemistry
GeneralUsing your knowledge of the hard soft acid base HSAB theory predict with reasoning whether you would expect the following reactions to occur i Fe acac 3 Fe Fe acac 3 Fe acac is the acetylacetonate anion ii Ni CO 4 2 e Ni CO 4 iii Hg OH 2 Mn SH 2 Hg SH 2 Mn OH 2 iv AgCl 2 CN Ag CN 2 2 CI

Physical Chemistry
EnergeticsAtmospheric O is measured in Dobson units 1 unit 2 69 x 1016 molecules O above each squared centimeter of Earth s surface Dobson thickness in hundredths of a millimeter that the O column would occupy if it were compressed to I atm at 0 C The graph shows variations in ozone concentration as a function of latitude and season Using an absorption cross section of 2 5 x 10 19 cm calculate the transmittance in the winter and the summer at 30 50 N latitude at which 0 varies from 290 to 350 Dobson units Twinter Tsummer Total ozone Dobson units 380 350 320 290 260 230 1989 1990 1991 30 N 50 N 10 N 30 N 10 N 10 S By what percentage is the ultraviolet transmittance greater in winter than in summer 1992 1993 Year Harris Lucy Quantitative Chemical Analysis 10e 2020 W H Freeman and Company Variation in atmospheric ozone at different latitudes Data from P S Zurer Chem Eng News 24 May 1993 p 8

Physical Chemistry
Chemical BondingThe volume of 0 0168 mol of O2 obtained by decomposition of KClO3 and collected by displacement of water is 428 mL at a pressure of 754 mm Hg at 25 C The pressure of water vapour at 25 C is OA 18 mm Hg OB 20 mm Hg C 22 mm Hg

Physical Chemistry
Electrochemistry1 20 2H O a e b OH E 0 4 V vs NHE Zn c OH Zn OH d e E 1 25 V vs NHE Please determine the value of a b c d and calculate the maximum energy that can be generate by of Zn air battery use tne units in J per 1 mole Zn

Physical Chemistry
Atomic StructureAssertion A In F molecule F F bond is weak Reason R Small size of F atom a A and R both are correct and reason R is correct explanation of A b A and R both are correct but reason R is not correct explanation of A

Physical Chemistry
Electrochemistry4 Balance the following reaction in acidic condition and compute for the AG O2 g Cu s Cu H O Electrode potentials for the half reaction are 1 23 V and 0 34 V for the Oxygen half reaction an Copper half reaction respectively

Physical Chemistry
GeneralYou are examining a diatom sample using a BH2 polarizing light microscope with the 0 40 numerical aperture 20X magnification objective lens in air but you re not able to resolve the fine details of the sample with these settings Describe three different specific methods that you can use to improve the resolution of detail of this sample Explain in detail how each of these methods works to improve resolution of detail You can assume that the microscope has already been set up for K hler illumination

Physical Chemistry
GeneralFor reversible Galvanic cell thermodynamic parameters are given as AG Tp AH TAS 4 AG Tp nFEcell nF de cell dT AS Wusefull AG T P For given cell Pt H HCI AgCl Ag variation of e m f of the cell with temperature is given as Ecell in volt 0 4 2 10 T T temperature in Kelvin Use 1F 96500 C P For given cell magnitude of entropy change occured for production of one mole of Ag in J K at 27 C will be