Physical Chemistry Questions
The best high school and college tutors are just a click away, 24×7! Pick a subject, ask a question, and get a detailed, handwritten solution personalized for you in minutes. We cover Math, Physics, Chemistry & Biology.

Physical Chemistry
Energetics47 Two moles of helium gas are taken over the cycle ABCDA as shown below in the P T diagram Assume the gas to be ideal and R is gas constant 400R 2x105 P Pascal 1x 105 A 416R 300 K Floor B C 500 K 693 Temp K Now match List I with List II and select the options given below List I PY Magnitude of work done by the gas in taking from A to B Q Magnitude of work done by the gas in taking from B C R Magnitude of work done on the gas in taking from D A S 2x2 V 2 0 0121 30 Clash 300 k Magnitude of the net heat absorbed evolved in the cycle ABCDA 1 693 R 2 277 R 3 400 R 4 416 R ISOR List II 24 63 1 S

Physical Chemistry
EnergeticsA aq B aq AB aq The reaction shown above is carried out in a coffee cup calorimeter The reaction occurs in 350 0 of solution initially at 22 6 C The temperature of the solution increases to 50 7 C when the reaction is carried out using 0 495 mol of A Calculate the enthalpy change for the reaction AH i kJ mol Enter a numerical value in the box provided in 3 significant figures including the proper sign Assume that the specific heat capacity of the solution is 4 18 J g C

Physical Chemistry
Chemical kineticsThe partial pressure of hydrogen in a fla containing 2 g H and 32 g SO is 1 1 16th of total pressure 2 1 9th of total pressure 3 2 3rd of total pressure 4 1 8th of total pressure

Physical Chemistry
Equilibrium10 g sample of gas liquor NH salt is boiled with NaOH and the resulting NH3 is passed into 60 ml of 0 90 N H SO4 Excess H SO4 required 10cm of 0 40 N NaOH What is the of NH3 in gas liquor A 8 5 C 9 5 B 7 5 D 10 5

Physical Chemistry
EnergeticsH g C H4 g C H6 g AG 98 0 kJ and AS 120 7 J K at 323 K and 1 atm The maximum amount of work that could be done by this reaction when 2 10 moles of H g react at standard conditions at this temperaturo in 22k L

Physical Chemistry
Atomic StructureSodium emits yellow light with a frequency of 5 09x10 4 Hz when electrons transition from 3p to 3s orbitals I Calculate the energy difference in J between these two orbitals using sections 1 and 2 of the data the booklet

Physical Chemistry
Chemical kinetics0 The results given in the below table were obtained during kinetic studies of the following reaction 2A B C D 1 Experiment A moll 1 0 1 II 0 1 III 0 2 IV V X 0 3 B molL 0 1 0 2 0 1 0 2 Y Initial rate molL min 6 00 x 10 3 2 40 x 10 2 1 20 x 10 2 7 20 x 10 2 2 88 x 107 X and Y in the given table are respectively A 0 3 0 4 B 0 4 0 3 C 0 4 0 4 D 0 3 0 3

Physical Chemistry
Chemical Bonding3 A colourless solid X on heating evolved CO and also gave a white residue soluble in water Residue also gave CO when treated with dilute acid X is a Li CO3 c Ca HCO3 2 b CaCO3 d NaHCO3

Physical Chemistry
Chemical BondingNote this is a multi select question Isostoichiometric species have the same molecular formula but different electronic configuration Select ALL the isostoichiometric species from the list below ON N N N O H2 H H 111 Q Q no 2

Physical Chemistry
ElectrochemistryThe following cell has a potential of 0 55 V at 25 Pt s H 1 atm H M Cl 1 M Hg Cl s Hg 1 What is the pH of the solution in the anodic chamber E 0 28 V 1 457 Cl Hg Cl Hg 2 14 07 3 0 15 4 7 15

Physical Chemistry
GeneralIn the given process which oxidation state is more stable MIE 7 9 eV M M 42 M2 IE 15 5 eV 1 M The electronic configuration of some neutral atoms are given below 1961 2 M 2 3 Both

Physical Chemistry
GeneralWhat is the product of 1 and 4th reactions Which reaction does not produce ammonia AH B TH 3I H DUI 3THI P Pfa T a NH4 I KOH b N 3H A Fe Mo c NH4 Cr O d Mr N HCL og

Physical Chemistry
EquilibriumOne mole of acetic acid and one mole of methyl alcohol were mixed at 298 K At equilibrium 0 333 mole of acetic acid was found to be unreacted Calculate the equilibrium constant for the reaction

Physical Chemistry
GeneralThe degree of inhibition a by a 1 point inhibitor is competitive obtained from O O O O O measurement of Vmax measurement of the y intercept on a Limeweaver Burke Plot measurement of KM is unrelated to the binding affinity of the inhibitor to the enzyme

Physical Chemistry
Chemical kineticsWhich condition defines a reaction at equilibrium O The concentration of products is equal to the concentration of reactants The reactions forming products and reactants come to a stop The concentration of products is greater than the concentration of reactants The rate of the forward reaction is equal to the rate of the reverse reaction The concentration of reactants is greater than the concentration of products

Physical Chemistry
Chemical kineticsThe rate law for the oxidation of Nitric oxide 2NO O NO is given by rate KNO O If the volume of reaction vessel is reduced to 1 4 th of its original volume then how will the rate of reaction will change consider initial rate and final rate as R and R

Physical Chemistry
Energetics4 Calculate the change in the entropies of the system and the surroundings and the total change in entropy when a sample of nitrogen gas of mass 14 g at 298 K and 1 00 bar doubles its volume in a an isothermal reversible expansion b an isothermal 0 and c an adiabatic irreversible expansion against pext reversible expansion
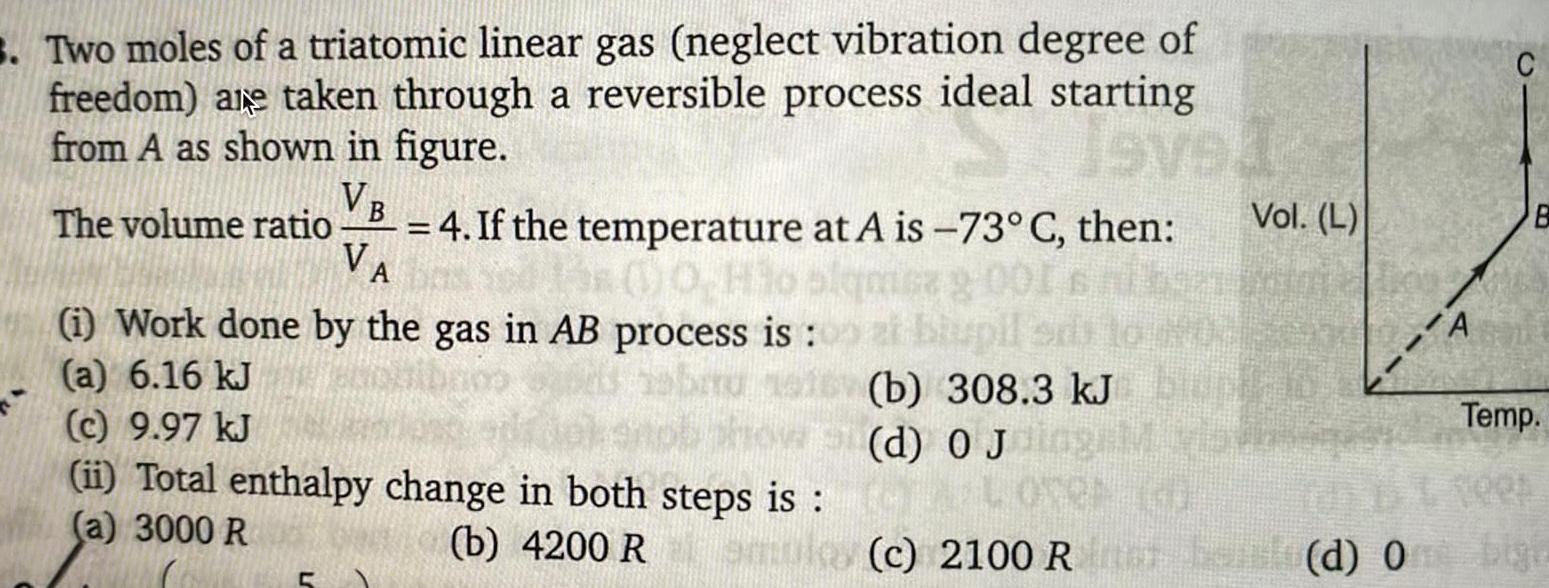
Physical Chemistry
Gaseous and liquid states3 Two moles of a triatomic linear gas neglect vibration degree of freedom are taken through a reversible process ideal starting from A as shown in figure Seved VB The volume ratio sa 9 00 VA 4 If the temperature at A is 73 C then 0 Ho i Work done by the gas in AB process is a 6 16 kJ c 9 97 kJ ii Total enthalpy change in both steps is a 3000 R Vol L al biupil b 308 3 kJ BUT d 0 Jolag vis CA LOVER b 4200 Romulos c 2100 Ron A B Temp Tees belo d One bigi

Physical Chemistry
EquilibriumA chemical reaction is carried out by starting with one mole each of H and I in closed vessel at 717 K No more change in concentration was observed after 1 56 mole of HI is formed If we start the reaction with 2 mole of HI under similar conditions how many moles of H and I will be formed

Physical Chemistry
GeneralPEI can be degraded by a microbe called Id eonella Sakaiensis 1 What is the relation between Heat co nditions Temperature of the environment a nd the time of degrdation 2 What are the by products of this degr adation process Are they beneficial for th e environment

Physical Chemistry
ElectrochemistryCalculate the equilibrium constant for the reaction 3Mg2 2Al 3Mg 2Al For Mg 2e Mg k E 2 363 For Al 3e Al k E 1 662 Cr solution is obtained by electrolysis of Cr solution In 500 mL of 0 15 M Cr solution how long would it take to reduce Cr to Cr using a current of 0 158 A The potential of the DKE MgA2 Mg2 9 62 x 10 M Mg ISE ion selective electrode Cell is 0 367V When the Mg2 solution in the cell is replaced with an unknown Mg2 solution the cell potential becomes 0 244 V What is the concentration of this Mg2 solution

Physical Chemistry
Generalg 16 Enthalpy change for the reaction 4 Hg 2H is 869 6 kJ The dissociation energy of H H AIPMT Prelims 2011 bond is 1 217 4 kJ 2 434 8 kJ 3 869 6 kJ 134 8 k

Physical Chemistry
GeneralWho among the following scientists is not related to production and detection of electromagnetic waves G Marconi H Hertz min sec J C Bose

Physical Chemistry
EnergeticsConsider the reaction A s B s C g anc the following thermodynamic data at 298 K Substance AH kJ mol AG kJ mol 1034 33 1020 02 845 02 650 93 462 83 576 77 A s B s C g Pc at 1230 44 K in bar

Physical Chemistry
SolutionsPls give detailed solution A 0 5 dm flask contains gas A and 2 dm3 flask contains gas B at the same temperature If density of A 4 g dm that of B 2 g dm and molar mass of A is half of that of B then the ratio of pressure exerted by gas is PA Pa PA 2 Pa P 0 5 11 4

Physical Chemistry
SolutionsQ33 Assume three samples of juices A B and C have glucose as the only sugar present in them The concentration of sample A B and C are 0 1 M 0 5 M and 0 2 M respectively Freezing point will be highest for the fruit juice have same freezing point a A b B c C d all Q41 Molecule following r a 2 Brom Q42 A compou

Physical Chemistry
Gaseous and liquid statesWhich of the following is correct about Contact process for the manufacture of sulphuric acid a V 05 is used for catalytic oxidation of SO to SO3 b SO2 is produced which is absorbed in water c SO3 is directly absorbed in water SO3 is absorbed in concentrated sulphuric acid d Only One Correct Answer

Physical Chemistry
EnergeticsIE and IE of Mg are 178 and 348 kcal mol respectively The energy required for the reaction Mg g Mg 2e is 2 1 a 170 kcal mol c 170 kcal mol b 526 kcal mol d 525 kcal mol

Physical Chemistry
ElectrochemistryFor an electrode reaction Mn aq ne M s The electrode potential at any concentration measured with respect to standard hydrogen electrode can be represented by Nernst equation as E Mn M but concentration of solid M is taken as unity so we 1 have E M M RT In Mn E Mn M nF Q For given reaction of electrode Cl g 2e 2Cl aq Nernst equation is given as E In Mn M A Nernst equation is given as Only One Correct Answer Q For given reaction of electrode Cl g 2e 2Cl aq Ecl Cl EC Cl B Ecl Cl E IVI IVI C Ecl Cl EC1 C1 Cl g 2e 2Cl aq Only One Correct Answer Cl Cl C Ech Cl Nernst equation is given as A Ecl Cl EC1 Cl RT M nF Mn RT 2F Ecl cl D Ech c Ech c B EC Cl EC C 2 1 RT In RT CI RI In F Pcl2 RT Ecl ct In CI 2F In CI RT CI 1 In 2F PC12 TUL RT 2F In RT CI 1 In 2F PC12 CI PC12 CU 12 PC12 AVI

Physical Chemistry
EquilibriumCalculate the equilibrium concentration of Mg2 and F 1 1 0L of 6 0 10 M Mg NO3 2 is added to 2 0L of 3 0 10 M NaF Calculate the equilibrium concentration of Mg2 and F Kap of MgF2 4 0x10 5 pts

Physical Chemistry
Energetics31 The standard entropies of N 9 H g and NH g are 191 5 130 5 192 6 JK 1 mol 1 The value of AS of formation of ammonia is 1 98 9 JK 1 mol 1 3 129 4 JK 1 mol 1 2 Zero 4 29 4 JK 1 mol 1

Physical Chemistry
GeneralColumn l contains pair of cations and column II contains the basis on which the cations can be distinguished Column l Q1 Pb2 and Bi Q2 Zn2 and Fe Q3 Cu and Ni Q4 Cu2 and A1 Column II A1 Excess NH4OH A2 Excess NaOH A3 dmg A4 NH4OH H S A5 H S HCI

Physical Chemistry
GeneralWhen the following skeletal equation is balanced under acidic conditions what are the coefficients of the species shown 12 Fe 103 Fe Water appears in the balanced equation as a reactant product neither with a coefficient of Enter O for neither Which species is the reducing agent

Physical Chemistry
Solid stateWhich positions of lattice are occupied by chloride ions and sodium ions respectively in Rock salt structure Only One Correct Answer A Corners and edge centers B C Octahedral void and corners Corners and face centers

Physical Chemistry
EquilibriumFe s CO g For a reaction FeO s CO g the value of K at 1373 K is 0 505 If the reaction mixture has 1 mole each of Fe and FeO 1 5 atm CO and 0 6 atm CO in which direction will the reaction go

Physical Chemistry
SolutionsWhich of the following statements are correct I Apure substance has fixed melting and boiling points II If a liquid is impure it will boil over a range of temperatures but will freeze at a fixed temperature III If the pressure acting on a liquid is increased the boiling point will increase IV A pure orange juice will have a fixed boiling point A I and III only B I and II only C III and IV only D I II and IV only

Physical Chemistry
Electrochemistry5 The following galvanic cell has a potential E of 0 98 V at 298 K Zn s ZnX4 0 010 M X 2 00 M Zn 2 00 M Zn s a Write the balanced equation for the cell reaction and calculate E

Physical Chemistry
SolutionsA 30 L container is found to have 20 L acetone 1 at 780 mm of Hg pressure If 2 L of liquid acetone is taken out then calculate the total gaseous pressure approx inside the container Vapour pressure of acetone at given condition is 400 mm of Hg

Physical Chemistry
GeneralA 1 00 L sample of a mixture of methane gas and oxygen measured at 25 C and 740 torr pressure was allowed to react at constant pressure in a calorimeter which together with its contents had a heat capacity of 1260 cal K The complete combustion of the methane to carbon dioxide and liquid water caused a temperature rise in the calorimeter of 0 667 K What was the mol percent of CH4 in the original mixture AH comb for CH g 210 8 kcal mol

Physical Chemistry
Chemical kineticsFor a given reaction AH 35 5 kJ mol and AS 83 6 JK 1 mol 1 The reaction is spontaneous at Assume that AH and AS do not vary with temperature at high Temp NEET 2017 1 T 425 K 2 T 425 K 3 All temperatures 4 T 298 K

Physical Chemistry
GeneralSelect the correct graph representing the following equilibrium if initially equal moles of NH3 and O2 are taken in reaction vessel 4NH3 g 50 g 4NO g 6H O g Conc Conc Conc Conc NH H O Time 0 H O Time NO H O Time 0 0 NO Time NH NO 0 NH H O NH NO

Physical Chemistry
ElectrochemistryCopper sulphate solution 250 ml was electrolysed using a platinum anode and a copper cathode A constant current of 2 mA was passed for 16 minutes It was found that after electrolysis the absorbance of the solution was reduced to 50 of its original value Calculate the concentration of copper sulphate in the solution to begin with A B C D 7 95 x 10 5M 7 95 10 4M 7 95 10 M 7 95 10 2M

Physical Chemistry
Gaseous and liquid statesI know that this is something we practically observe that all gases stay diffused throughout the s pace despite their molecular weight but I am not able to understand why this is so because if u see then in cold air and warm air cold air being denser settles at bottom whereas warm air remain s on top then why isn t the same principle applicable for molecular masses i e one with higher molecular mass comes to bottom and the lighter one stays at top I read an available answer on it utor and the teacher says that molecular mass doesn t show heaviness of a gas But if this was so then why do we say that lighter gases diffuse out faster out of an orifice than heavier ones The mixture of three gases X Y and Z is enclosed in a closed vessel at constant temperature Molecular weight of X is the highest and that of Y is the least When equilibrium is established the 1 Gas X will be more at bottom 2 Gas Y will be more at top 3 Gas X Y Z are homogeneously present 4 Gas Y will be more at bottom ns

Physical Chemistry
GeneralA new assay method is being evaluated in comparison to an existing gold standard method Repeated runs of the new method show assay points that are closely clustered together However the mean measurement from the new assay is significantly different from the mean measurement of the same samples with the gold standard method Which of the following statements could account for this observation There is likely to be an interfering chemical in the new assay method that is affecting the accuracy of the method There are random errors during performance of the new assay method due to operator error The new assay has very little systematic error None of the above

Physical Chemistry
Equilibrium2NO g at Consider the reaction N O g 373 K Suppose at a particular moment concentrations of N O4 and NO are 1 mol L each In which direction the reaction will proceed to establish equilibrium K for the reaction is 0 36 2

Physical Chemistry
Chemical BondingConsider the following interaction of atomic orbitals 8 0 8 Answer 00 01 b 8 8 Out of the above combination X Number of non bonding combinations Y Number of combinations that produce only antibonding molecular orbital Z Number of combinations that produce only bonding molecular orbital V Number of combinations that produce bonding T molecular orbital W Number of combinations that produce antibonding T molecular orbital The value of X Y Z V Wis 1 e 8 X 02 02 c 3 1 X X X 04 04 05 06 E CO 07 7

Physical Chemistry
Atomic StructureSpider Man No Way Home 3D Hindi Hindi UA Hindi 3D 09 30 AM 12 45 PM 03 05 PM 10 55 P Spider Man No Way Home Hindi Hindi UA Hindi 2D
Physical Chemistry
GeneralAccording to the 2nd Law of Thermodynamics for a reaction to be spontaneous i e proceed naturally which MUST be true a AS system 0 b AS surroundings 0 O c AS system 0 d AS universe 0 O a b and d

Physical Chemistry
Chemical BondingThe first AH and second AH ionisation enthalpies in kJ mol and the electron gain enthalpy AH in kJ mol of the elements I II eg III IV and V are given below Elements A H AgH I 520 60 II 419 48 III 1681 328 IV 1008 295 V 2372 48 The most reactive and the least reactive element amongst these are respectively a I and V c II and V A H 7300 3051 3374 1846 5251 b V and II d IV and V

Physical Chemistry
GeneralQ13 In a regular binary A B solid solution the molar fraction of component B is 0 4 and its activity is 0 5 at temperature of 700 K What is the lowest temperature that can prevent the phase separation in the regular solid solution